Measurement of Courtship Behavior in Drosophila melanogaster
- Department of Biology, Brandeis University, Waltham, MA 02454, USA
- National Center for Behavioral Genomics, Brandeis University, Waltham, MA 02454, USA
- Volen Center for Complex Systems, Brandeis University, Waltham, MA 02454, USA
- ↵1Corresponding author (aki{at}brandeis.edu)
INTRODUCTION
In Drosophila melanogaster, as in many other animals, courtship is a series of stereotypical behaviors carried out by a male responding to multimodal signals. Because different experimental conditions can engage distinct sensory modalities that affect male behavior, courtship experiments need to be carefully designed. There are several ways to manipulate sensory inputs to the test male. This protocol describes methods for designing and conducting experiments that measure the various parameters of courtship behavior.
RELATED INFORMATION
Male Drosophila initiate courtship behaviors in response to various sensory inputs (see Fig. 1, Movie 1). Vision is the most prominent sensation that triggers courtship behavior in males. Once a male finds a female, he immediately starts courting with orientation behavior (turning toward and chasing the moving female); this behavior is often accompanied by slight shaking of both wings or body wagging. When it is dark (such that visual cues are not available), the male is still able to find the female using other cues. These cues (olfaction and mechanosensation) work only at a short distance, and so the male needs more searching time to find the female. Thus, courtship latency (see Discussion) is increased in low light.
The stimulatory sensory inputs that progress courtship behavior in male Drosophila. Arrows indicate a strong contribution to courtship drive.
Following orientation, the male touches the female’s body with his foreleg (tapping). The physical contact of tapping contributes to the identification of the sex and species of the target by the male. If the male senses female sex pheromones via the gustatory organs on his foreleg, courtship is propelled to the next step, wing vibration. Wing vibration consists of repeated flicking of a wing that produces a species-specific courtship song. A “good” courtship song makes the female receptive to copulation. Audition is also important for males themselves; a male listens to and fine-tunes his own song, and courtship song is known to boost the courtship enthusiasm of the “singer” and other males that hear the song.
After or during wing vibration, the male extends his proboscis and licks the female’s genitalia (licking). Following licking, the male tries to grab the female body with his forelegs and curls the tip of his abdomen (attempting copulation). If the female is receptive, she spreads both her wings to allow the male to mount (courtship success). Usually the male repeats the entire display several times, until the female accepts his mounting. Once the mounting is successful, both the male and female stay still for 10 to 20 min while the male ejaculates seminal fluid into the female. For more information, see Spieth (1974).
MATERIALS
Reagents
Drosophila melanogaster, naive males and target females (wild-type, Canton-S or Oregon-R)
Ethanol
Food media
Equipment
Aspirator, mouth
Courtship chamber (see Step 5)
The courtship chamber should be washed and cleaned with ethanol between uses to eliminate residual fly scents. Cell size can have a big influence on which sensory modality the male uses to find the female. The bigger the chamber, the more the male requires vision and/or female movement to start courtship.
Filter paper, Whatman ashless #42
Place water-soaked filter paper in each cell of the courtship chamber.
Incubator with a light/dark cycle, set to 25ºC
Light, white (for bright conditions) or dim-red (>700 nm, for dark conditions)
Scissors, fine (optional; see Step 4)
Test tubes
Vials
Video recording equipment
METHOD
Experimental Design
Courtship experiments should be carefully designed because different conditions can engage distinct sensory modalities and thus affect behavior.
-
1. Sensory inputs to the test male may be manipulated using the following methods:
-
i. To eliminate visual input, use dark or dim-red light conditions.
This manipulation is useful when investigating chemosensory mechanisms because visual cues are very strong courtship stimuli and they often mask subtle changes in other modalities (Joiner and Griffith 2000).
-
ii. To investigate the visual contribution to courtship, use a pheromone-less decoy, whose cuticular hydrocarbons (sex pheromones) have been washed off with hexane, as a courtship target.
-
iii. To block active movements by the female, use anesthesia or decapitation; see Step 4.
Although a Drosophila female doesn’t demonstrate an active courtship display, her reaction to male courtship (acceptance or rejection) affects the performance of the courting male. Using a motionless target not only reduces mechanosensory input to a male, but also prevents copulation, allowing the investigator to have consistent observation periods for each experiment (e.g., 10 min; see Discussion).
-
Preparing Adult Flies
Use sexually mature males and females (4 or 5 d old) for experiments.
-
2. Raise flies at 25°C with a light/dark (L/D) cycle. Maintain room humidity at 70%.
-
3. Collect naive males on the day of eclosion and keep them in individual test tubes (with no pretest social experience) in a 25°C incubator with an L/D cycle. To avoid ethanol exposure, use autoclaved food without sprinkled yeast granules.
-
4. Target females should be stored in groups (e.g., 30 females per vial). To prepare immobilized females, anesthetize them with CO2 or ether, or decapitate them with fine scissors immediately before the experiment.
Mild CO2 exposure for 50 min causes immobilization for the following 10 min. Some investigators prefer to avoid the use of ether because ether fumes may be emitted by the female.
Courtship Observation
-
5. Assemble a courtship chamber containing wet filter paper in each cell to maintain humidity.
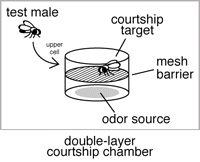
A double-layered chamber, in which a mesh barrier blocks physical contact with the odor source, can be used to evaluate the involvement of olfaction separately from gustatory function (see Fig. 2 and Ejima et al. 2005).
-
6. Label the chamber with the date and fly ID numbers (do not include genotype or specific manipulation).
-
7. Transfer a test male (without anesthesia) to each cell of the chamber using a mouth aspirator.
-
8. Allow the males to acclimate to the chamber for 5 min.
-
9. Using a mouth aspirator, introduce a target female to each cell and immediately start video recording (10 min).
-
10. Analyze the recording. During the behavioral analysis, the observer should refer to only the fly ID numbers and must be blind to the other experimental information (e.g., genotype or specific manipulation).
DISCUSSION
Multiple parameters may be used in the courtship analysis. The courtship index (CI) is broadly used to represent the overall courtship enthusiasm of the male. It is measured as the total duration of the male’s performance of any steps of courtship and calculated as a fraction of the observation period (i.e., 10 min or until copulation is achieved). The courtship levels and display patterns are very sensitive to the experimental conditions, as mentioned above. For example, the CI for a wild-type male (Canton-S) ranges from 0.3 (in a large arena under dark conditions) to 0.9 (in a small arena under bright conditions). The wing extension index (WEI, also known as wing vibration index or WVI) is the total duration of wing vibration, also calculated as a fraction of observation period. WEI corresponds to a portion of CI; however it does not always show a linear correlation with CI (Villela et al. 2005). A male may retain the same level of CI but change his behavioral pattern by spending more time in one display (e.g., chasing) and less time in another (e.g., wing vibration). The precise character of the courtship pattern can be described by numbers of each courtship event (orientation, tapping, licking, or attempting copulation), together with WEI.
Courtship latency is the time lag before performance of the first courtship behavior (orientation) after pairing. Both the chamber (arena) size and the light condition affect courtship latency. The bigger the chamber and the lower the light intensity, the more time the male requires to find a target female and start courtship. Wing vibration latency is the time lag before the first display of wing vibration after pairing. Because the wing vibration is first performed after tapping behavior, a defect in gustation (tasting target pheromones with the tarsi) is expected to cause prolonged wing vibration latency, while leaving courtship latency unaffected.
Copulation latency is the time lag before successful mounting after pairing. A parameter similar to copulation latency, mating success, can be measured as the number of pairs that achieved successful copulation during a certain period (e.g., 30 min). Copulation latency and mating success represent not only the courtship enthusiasm of the male but also the basal receptivity of the female and the quality of the male’s performance (which can enhance the female’s receptivity). For example, the deaf mutant beethoven, which produces a bad courtship song (through abnormal wing vibration frequency), has a normal CI but doesn’t succeed in mating as well as a wild-type male (Eberl et al. 1997).
Courtship levels and behavior patterns can be significantly affected by the genetic background of the test flies. For each assay, an appropriate control strain must be prepared. Out-crossing is an option. Once a mutant strain is established, the genomic background can be exchanged by crossing the strain to a wild-type stock (e.g., Canton-S, “cantonizing”) for more than five generations, which allows the background donor strain to be used as a control. For a P-element imprecise excision mutant, a precise excision line can be a good control. For an experimental male made using the GAL4-UAS system, uni-transgenic flies (GAL4/+ and UAS/+ heterozygotes) must be examined for each assay. Lastly, it should be noted that ectopic expression of white (mini-w+), a marker gene commonly used for making transgenic flies, can trigger high levels of male-male courtship (Zhang and Odenwald 1995). Hing and Carlson (1996) showed that the homosexual courtship driven by mini-w+ expression was significantly reduced by elimination of visual cues, suggesting that the gene product of mini-w+ disturbed some part of the visual system. Therefore, observing behavior in dark or dim-red light conditions is useful for minimizing the mini-w+ effect in a courtship assay.