Desorption Electrospray Ionization (DESI) Analysis of Intact Proteins/Oligopeptides
This protocol was adapted from “Desorption Electrospray Ionization: Proteomics Studies by a Method that Bridges ESI and MALDI,” Chapter 6, in Proteomics: Methods Express (eds. O’Connor and Hames). Scion Publishing Ltd., Oxfordshire, UK, 2007.INTRODUCTION
Desorption electrospray ionization (DESI) is amenable to the study of intact proteins in complex mixtures, including blood or other biological media. Intact proteins can be desorbed and ionized from the surface under gentle (soft) conditions to produce compact conformations of the protein. A procedure for DESI analysis of intact proteins and oligopeptides using mass spectrometry (MS) is described here. DESI-MS is an emerging technique with great promise, but its application range is still being investigated. Therefore, the protocol presented here provides general procedures used for the applications that have been investigated so far. Optimal ion source parameters and surface types may vary depending on the application.
RELATED INFORMATION
An introduction to DESI instrumentation, methods, and applications is provided in the CSH Protocols article Desorption Electrospray Ionization: Proteomics Studies by a Method that Bridges ESI and MALDI (this issue). Protocols for Desorption Electrospray Ionization (DESI) Analysis of Tryptic Digests/Peptides and In Situ Desorption Electrospray Ionization (DESI) Analysis of Tissue Sections are also available (this issue). The use of DESI-MS for analysis of protein structure is described by Myung et al. (2006).
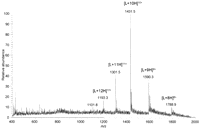
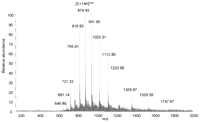
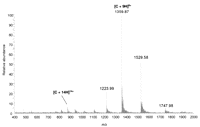
As an example of the type and quality of data obtained on intact pure proteins, Figure 1 shows the DESI mass spectrum of 1 pmol of lysozyme prepared by dissolution in methanol:H2O (1:1) solvent and deposition onto a polytetrafluoroethylene (PTFE) surface. Both the composition of the solvent in the spray and that used to dissolve the analyte affect the appearance of the mass spectrum. For example, the use of acidified aqueous/organic solvents for deposition of the analyte produces results similar to the results obtained with ESI of the same sample where higher charge states appear in the mass spectrum, indicating an unfolded or partially unfolded conformation of the protein. The mass spectra in Figure 2 show the data obtained when cytochrome c is dissolved in methanol:H2O:acetic acid (50:48:2) as opposed to methanol:H2O (50:50), as shown in Figure 3.
DESI analysis of intact protein. DESI mass spectrum of 1 pmol of lysozyme (L) deposited onto a PTFE surface. Methanol:H2O (1:1) was used as the spray solvent at a flow rate of 5 μL/min. (Reprinted with permission, © 2007 Scion Publishing Ltd.)
DESI mass spectrum of cytochrome c. DESI mass spectrum of 1 pmol of cytochrome c (C) dissolved in methanol:H2O:acetic acid (50:48:2) and deposited onto a PTFE surface. Methanol:H2O (1:1) was used as the spray solvent at a flow rate of 5 μL/min. (Reprinted with permission, © 2007 Scion Publishing Ltd.)
MATERIALS
Reagents
Protein of interest (1-100 ng/mL)
Any solvent system can be used for deposition, as long as the protein is soluble in the selected solvent; see Step 1.
Spray solvent, such as one of the following:
Do not use nonvolatile buffers or high concentrations of acid (e.g., acetic acid).
Equipment
Mass spectrometer equipped with a DESI ion source
Pipette tips
Surface slides (polymethyl methacrylate [PMMA], PTFE, or glass)
METHOD
-
1. Deposit a 1-μL aliquot of a solution containing the protein of interest onto a PTFE, PMMA, or glass slide.
The unfolding and thus the charge state distribution of the protein ions depend on the composition of the solvent system used for deposition.
-
2. Allow the protein sample spot to dry.
-
3. Expose the dried sample to pneumatically assisted microelectrospray using a DESI ion source coupled to a mass spectrometer; typical parameters used for analysis of proteins and oligopeptides are as follows:
Parameter Value Spray tip-to-surface distance 1-2 mm Incident angle of spray 70°-80° Spray tip-to-MS inlet distance 3-5 mm Collection angle 0°-5° Solvent flow rate 1-5 μL/min Nebulizing gas linear velocity 300-400 m/sec Spray high voltage 4-6 kV Surface temperature 30°C-80°C See Troubleshooting.
TROUBLESHOOTING
Problem: There are no surface-originated ions in the spectra.
[Step 3]
Solution: A possible cause of this is that the sprayed droplets and ions are not reaching the surface because of incorrect spray parameters (i.e., the volumetric flow rate is too low or there is no applied high voltage) or surface charging effects. Always check the spray pattern on the surface. If the spray is not visible on a glass surface, check to ensure that the solvent syringe pump is on and that there are no blockages in the solvent delivery line that would restrict flow.
Problem: No signal is detected.
[Step 3]
Solution: There may be no spray present, or the sprayed species are being deflected by the charged surface.
Try the following:
-
1. Increase solvent and gas flow rates.
-
2. If there is no change, remove the surface and check whether the spray produces ions. Test the spray with 10 mg/mL bovine cytochrome c in 10 mM aqueous ammonium acetate. The test solution should give a narrow charge state distribution of the protein, with a main charge state of +7 or +8. If there are no ions, check whether the spray tip (or solvent line) is clogged. If the cytochrome c spectra are full of adducts, change the spray tip.
Problem: There is excessive adduct formation.
[Step 3]
Solution: This may be caused by insufficient desolvation due to the presence of large droplets or contamination of the surface. Try the following:
-
1. Increase the gas flow rate and/or heat the surface.
-
2. Check whether the sample contains salts at high concentration. If the deposited sample contains >100 mM inorganic, nonvolatile salt, dilute it or remove the salts prior to deposition.
-
3. If a PMMA surface is used in combination with methanol (as solvent for the deposited sample), let the surface dry for an additional 10 min at 50°C.
Problem: A transient signal, not suitable for MS/MS, is observed.
[Step 3]
Solution: A possible cause of this is low surface concentration or lack of sample adhesion to the surface. The following are possible solutions:
-
1. Try a different surface material or a roughened surface.
-
2. Increase the surface concentration of the sample.
-
3. Increase the spray tip-to-surface distance.
-
4. Decrease the solvent flow rate.
Problem: Results exhibit strong suppression effects and poor spectral resolution.
[Step 3]
Solution: Possible causes are high surface concentration or an inappropriate solvent system. Try the following:
-
1. Decrease the surface concentration of the sample.
-
2. Change the solvent composition.