Measuring Interstitial Diffusion, Convection, and Binding Parameters in Mouse Tumors
Adapted from Live Cell Imaging, 2nd edition (ed. Goldman et al.). CSHL Press, Cold Spring Harbor, NY, USA, 2010.Abstract
Noninvasive techniques have been developed for the assessment of various parameters in normal and diseased tissues of mice. This protocol describes the measurement of extravascular parameters, including interstitial diffusion, convection, and binding parameters, in mouse tumors. A fluorescently labeled molecule of interest is infused into the tumor interstitium, followed by imaging using single-photon microscopy or multiphoton laser-scanning microscopy (MPLSM). Fluorescence recovery after photobleaching (FRAP) with spatial Fourier analysis is performed. To measure interstitial diffusion coefficients, multiphoton FRAP is performed.
MATERIALS
Reagents
Anesthetic: isofluorane 1%–3% inhalant (up to 5% for induction) or ketamine (80–100 mg/kg body weight [BW])/xylazine (5–10 mg/kg BW)
Fluorescently labeled molecule of interest such as fluorescein isothiocyanate (FITC)-labeled antibody, viruses, or nanoparticles
Tetramethylrhodamine (TMR)-labeled dextran (MW 2 × 106; 2 mg/mL) (for MPLSM only; see Step 1)
Equipment
Heating pad or similar device
Intravital microscopy workstation:
-
Conventional single-photon microscope
-
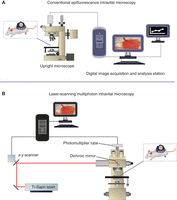
The conventional single-photon microscopy system (Fig. 1A) consists of a microscope (upright or inverted) equipped with transillumination and fluorescence epi-illumination, a flashlamp excitation device, a set of fluorescence filters, a motor-controlled filter wheel, a charge-coupled device (CCD) camera, a video monitor, a video recorder, and a frame-grabber board for image digitization. Alternatively, a spinning-disk confocal miscroscope or a laser-scanning confocal microscope can be used. Advanced techniques require additional equipment such as a motorized x–y stage with ±1.0-μm lateral resolution, an intensified CCD camera, a photomultiplier tube, and a dual-trace digital oscilloscope (Berk et al. 1997; Fukumura et al. 1997; Helmlinger et al. 1997; Jain et al. 2001, 2002).
-
-
Multiphoton laser-scanning microscope (MPLSM)
-
The MPLSM system (Fig. 1B) consists of a mode-locked Ti:sapphire laser and an x-y laser scanner purchased either as part of a multiphoton system or as a confocal system with further modifications for good infrared transmission. The laser beam first passes through a Pockels cell, which allows rapid (∼1-μsec) modulation of laser intensity and then is directed by the scan head into the side-entry or top-entry port of an upright epifluorescence microscope. Nondescanned photomultiplier tubes are used for imaging through significant depths of scattering tissue and should be introduced into the beam path via a dichroic beam splitter located in the beam path between the scan head and the objective lens (Denk et al. 1990; Brown et al. 2001). The system also requires a CCD camera, and a digital image and analysis station.
-
Intravital microscopy workstations. Mouse tumor models are observed using (A) conventional intravital microscopy or (B) MPLSM. With appropriate tracer molecules and/or engineered vectors/cells and computer-assisted image analysis, one can monitor tumor size, vessel density, vessel diameter, RBC velocity, leukocyte endothelial interaction, vascular permeability, tissue pO2, pH, gene promoter activity, enzyme activity, and delivery of drugs, including genes. (B: Adapted, with permission, from Brown et al. 2001.)
METHOD
-
Perform imaging procedures with the animal under appropriate anesthesia and with full approval by the Institutional Animal Care and Use Committee. During the procedure, maintain the animal’s core body temperature at 36°C–37°C using a heating pad or similar device.
-
1. Infuse a fluorescently labeled molecule into the tumor interstitium either via extravasation after intravenous injection or local low-pressure microinfusion (Netti et al. 2000; Pluen et al. 2001; Ramanujan et al. 2002). For MPLSM, highlight the vessels by injecting 100 μL of TMR-labeled dextran (MW 2 × 106, 10 mg/mL) into the tail vein.
-
For single-photon microscopy procedures, proceed to Step 2. For MPLSM, proceed to Step 5.
-
Single-Photon Microscopy Procedures
-
2. Bleach a subpopulation of the fluorescent molecules using a brief (approximately a millisecond) flash of focused laser light. During the bleaching flash, shutter the camera to avoid damage to the electronics.
-
3. Generate consecutive images of the bleached region via epifluorescence, and capture them on the CCD camera as unbleached fluorophore diffuses back into the bleached region.
-
4. Perform spatial Fourier analysis of the fluorescence recovery images as described previously (Chary and Jain 1989; Berk et al. 1993, 1997) to extract diffusion coefficients, convection velocity, and binding parameters.
MPLSM Procedures
-
5. Generate fluorescence with 840-nm excitation and collect it with 535DF40 (FITC) and 610DF75 (TMR) emission filters and a 570LP dichroic mirror.
-
6. Identify a location of interest from MPLSM images of blood vessels. Park the multiphoton focal volume at the location using the LSM control software. Bleach out a subpopulation of the fluorescent molecules using a brief (approximately a submillisecond) flash of high-intensity laser light.
-
7. Monitor the bleached region with the same laser beam but greatly attenuated. Use a multichannel scaler to record the recovery in fluorescence of the bleached region as unbleached fluorophores diffuse back into the bleached region.
-
8. Perform a mathematical analysis of the fluorescence recovery curve to extract the diffusion coefficient of the labeled molecules (Brown et al. 1999).
ACKNOWLEDGMENTS
The work described here was supported by grants from the National Institutes of Health, the National Science Foundation, the American Cancer Society, the United States Army, the National Foundation for Cancer Research, and the Whitaker Foundation.
- © 2013 Cold Spring Harbor Laboratory Press