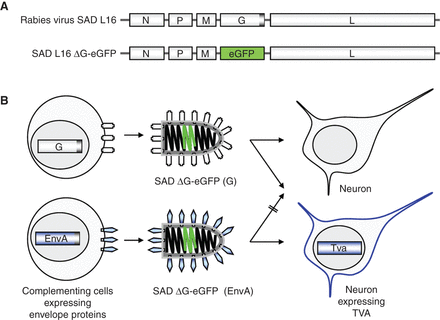
(A) Organization of the recombinant rabies virus (RV) RNA genome. The 12-kb RNA of RV (SAD L16) encodes five proteins—nucleoprotein (N), phosphoprotein (P), matrix protein (M), glycoprotein (G), and the RNA polymerase (L). N, P, L, and RNA are associated with the viral ribonucleoprotein (RNP); M forms a layer connecting the RNP with the lipid bilayer membrane; and G is a trimeric type I transmembrane spike protein mediating attachment of the virus to cell receptors and membrane fusion. SAD ΔG-eGFP lacks a G gene and encodes eGFP (enhanced green fluorescent protein) instead. (B) Pseudotyping of G gene-deficient virus. To yield virus particles able to infect cells, the G deficiency of SAD ΔG-eGFP must be complemented in cells expressing an analogous glycoprotein (i.e., a protein that binds to a receptor and mediates membrane fusion). For effective incorporation of heterologous proteins, such as the retroviral EnvA, their carboxy-terminal domains should be exchanged with that of G. As EnvA has a highly specific avian receptor (TVA), EnvA-pseudotyped RV infects only mammalian cells engineered to express TVA.










