Techniques to Distinguish Apoptosis from Necroptosis
- Maria Feoktistova1,4,
- Fredrik Wallberg2,4,
- Tencho Tenev2,4,
- Peter Geserick1,
- Martin Leverkus1,3,4,5 and
- Pascal Meier2,4,5
- 1Section of Molecular Dermatology, Department of Dermatology, Venereology, and Allergology, Medical Faculty Mannheim, University Heidelberg, 68167 Mannheim, Germany;
- 2The Breakthrough Toby Robins Breast Cancer Research Centre, Institute of Cancer Research, Chester Beatty Laboratories, London SW3 6JB, United Kingdom;
- 3Department of Dermatology & Allergology, University Hospital of RWTH Aachen University, 52074 Aachen, Germany
-
↵4 These authors contributed equally to this work.
Abstract
The processes by which cells die are as tightly regulated as those that govern cell growth and proliferation. Recent studies of the molecular pathways that regulate and execute cell death have uncovered a plethora of signaling cascades that lead to distinct modes of cell death, including “apoptosis,” “necrosis,” “autophagic cell death,” and “mitotic catastrophe.” Cells can readily switch from one form of death to another; therefore, it is vital to have the ability to monitor the form of death that cells are undergoing. A number of techniques are available that allow the detection of cell death and when combined with either knockdown approaches or inhibitors of specific signaling pathways, such as caspase or RIP kinase pathways, they allow the rapid dissection of divergent cell death pathways. However, techniques that reveal the end point of cell death cannot reconstruct the sequence of events that have led to death; therefore, they need to be complemented with methods that can distinguish all forms of cell death. Apoptotic cells frequently undergo secondary necrosis under in vitro culture conditions; therefore, novel methods relying on high-throughput time-lapse fluorescence video microscopy are necessary to provide temporal resolution to cell death events. Further, visualizing the assembly of multiprotein signaling hubs that can execute apoptosis or necroptosis helps to explore the underlying processes. Here we introduce a suite of techniques that reliably distinguish necrosis from apoptosis and secondary necrosis, and that enable investigation of signaling platforms capable of instructing apoptosis or necroptosis.
INTRODUCTION
Cells in the process of dying show different morphological features depending on the cell death process occurring. These features provide clues to the underlying molecular pathways that regulate and execute the different forms of cell death (Galluzzi et al. 2012). Although biochemical assays for monitoring cell death phenomena have become routine, a systematic methodology has not yet been adopted to distinguish apoptosis from necrosis (or necroptosis). Apoptosis is morphologically characterized by membrane blebbing, cell shrinkage and fragmentation, nuclear condensation, formation of apoptotic bodies, and activation of caspases (Galluzzi et al. 2012). The appearance of the major biochemical and morphological hallmarks of apoptosis are mediated by caspases (Luthi and Martin 2007). Activation of caspases can be triggered in response to immune-mediated signals via the “extrinsic” pathway, which is controlled by the death receptor family and their ligands. Alternatively, developmental cues and cellular stresses promote apoptosis via activation of the “intrinsic” pathway (Meier and Vousden 2007). Cells that die by apoptosis, at least in vivo, generally do not release their intracellular contents. However, apoptotic cells do not always die silently because they can trigger the secretion of chemotactic factors and other immunologically active proteins that can influence the immune response toward dying cells (Cullen et al. 2013). In contrast to apoptosis, necrosis is morphologically characterized by cell rounding, cytoplasmic swelling, presence of dilated organelles, and absence of caspase activation and chromatin condensation. Cells that die by necrosis spill their intracellular contents, and hence trigger an inflammatory response. Necroptosis refers to a regulated form of necrosis. It is biochemically defined as a form of cell death that is dependent on the serine–threonine kinase receptor-interacting protein 1 (RIPK1), RIPK3, and the mixed lineage kinase domain-like protein (MLKL) (Sun et al. 2012). This pathway plays important roles in a variety of physiological and pathological conditions, including development, response to tissue damage, and antiviral immunity (Vandenabeele et al. 2010).
STRATEGIES TO DISTINGUISH BETWEEN APOPTOTIC AND NECROPTOTIC CELL DEATH
Although the morphological features of apoptosis and necroptosis are clearly distinct, under tissue culture conditions apoptotic cells frequently undergo secondary necrosis (Berghe et al. 2010), which complicates the analysis of specific cell death modalities. Therefore, different strategies have to be used to analyze and distinguish apoptotic from necroptotic cell death. Chemical inhibitors of caspases, RIPK1, and MLKL are useful tools to determine whether cells die by apoptosis or necroptosis. Experimentally, apoptosis triggered by the intrinsic cell death pathway can be delayed (yet rarely completely blocked) by treatment with pan-caspase chemical inhibitors, such as N-benzyloxycarbonyl-Val-Ala-Asp-fluoromethylketone (zVAD-fmk) (Galluzzi et al. 2012) or quinolyl-valyl-O-methylaspartyl-[2,6-difluorophenoxy]-methyl-ketone (Q-VD-OPh) (Caserta et al. 2003). Although such pharmacological compounds are useful tools to inhibit caspases, it should be noted that the point of no return in mitochondria-mediated apoptosis is not caspase activation but irreversible dissipation of the mitochondrial membrane potential. Loss of mitochondrial membrane permeability (MOMP) leads to a release of toxic proteins from the intermembrane space into the cytosol that ultimately activates a positive feedback circuit that amplifies the apoptotic signal (Ekert et al. 2004). Activation of caspases, following MOMP, accelerates the execution phase of cell death. While chemical inhibition of caspases rarely confers long-term cytoprotective effects, or truly prevents cell death induced by death stimuli that funnel through mitochondria, zVAD-fmk and Q-VD-OPh can block most hallmarks of apoptosis. The cells still die, but via a different cell death modality, affecting morphology and immunogenicity of the dying cell. In contrast to cell death triggered via the mitochondrial pathway, zVAD-fmk and Q-VD-OPh can block most, if not all, apoptosis-inducing extrinsic signals. Thus, in combination with other techniques zVAD-fmk and Q-VD-OPh are useful tools to experimentally assess the modality of cell death, and to establish the relative contribution of caspases to cell death. The latter can be estimated by the extent of short-term (24–48-h) cytoprotection. While zVAD-fmk can delay apoptosis, it either has no effect or can even exacerbate necroptosis and can, therefore, be used as additional evidence for this type of cell death modality. For instance, treatment with zVAD-fmk stimulates necroptosis in L929 cells (Vandenabeele et al. 2010). This is because zVAD-fmk inhibits caspase-8/FLIPL heterodimers, which cleave and inactivate RIPK1 and RIPK3, thereby suppressing the formation of necroptotic complexes (Geserick et al. 2009; Kaiser et al. 2011; Oberst et al. 2011; Welz et al. 2011) (Fig. 1). Moreover, in L929 cells, treatment with zVAD-fmk drives autocrine production of tumor necrosis factor (TNF) (Hitomi et al. 2008), which promotes the assembly of necroptosis-inducing complexes.
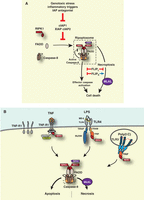
Cell death following formation of the RIPK1-dependent platform, also termed ripoptosome, complex-IIB, or necrosome. (A) cIAP1, cIAP2, and XIAP target RIPK1 and components of the ripoptosome (caspase-8 and cFLIPL) for Ub-mediated inactivation. Following genotoxic stress, cytokine signaling-induced depletion of cIAPs, or SMAC-mimetic (SM) treatment, cIAP1, cIAP2, and XIAP levels rapidly decline and/or are inactivated. This allows formation and accumulation of the ripoptosome. In the presence of high levels of RIPK3 this can lead to necroptosis. cFLIP also regulates ripoptosome-mediated cell death. cFLIPL prevents apoptosis and necroptosis, while FLIPS inhibits apoptosis but promotes necroptosis. (B) Under steady-state conditions, the majority of RIPK1 appears to be “inaccessible,” preventing it from binding to partner proteins. Cytokine receptor stimulation can convert a small fraction of RIPK1 into an “accessible,” binding competent configuration. In the presence of cIAPs and XIAP, binding competent RIPK1 is targeted for Ub-mediated inactivation, most likely via proteasomal degradation. Under conditions where IAP levels are low, however, unmodified and binding-competent RIPK1 accumulates and can form the ripoptosome. In the presence of high levels of cFLIPL, the ripoptosome is dissolved via caspase-8-cFLIPL-mediated cleavage of RIPK1. When cFLIPL levels are low, the ripoptosome can promote caspase-dependent or caspase-independent cell death.
Pharmacological inhibitors, such as necrostatin-1 (a RIPK1 inhibitor [Degterev et al. 2008]) and necrosulfonamide [an MLKL inhibitor (Sun et al. 2012)] are frequently used to suppress necroptosis. However, necrostatin-1 not only blocks necroptosis, but can also modulate RIPK1-mediated apoptosis (Tenev et al. 2011). Necrostatin-1 is, therefore, not a particularly specific tool to distinguish necroptosis from apoptosis, and conclusions cannot be drawn by using it as a stand-alone approach. Therefore, assays with these inhibitors need to be complemented with knockdown or knockout approaches targeting RIPK1, RIPK3, MLKL, or caspases to substantiate their contribution to apoptosis or necroptosis.
Overexpression of natural cell death regulators (e.g., cFLIP, XIAP, CrmA) and/or cellular inhibitors of apoptosis (cIAPs) proteins can also be used to analyze whether cells die by apoptosis or necroptosis. For example, ectopic expression of the short or long isoform of cFLIP blocks apoptosis induced by treatment with TNF and IAP-antagonists (Feoktistova et al. 2011; Oberst et al. 2011; Pop et al. 2011) (Fig. 1). However, in cells that harbor high levels of RIPK3 and that die by necroptosis, expression of the short isoform of cFLIP (cFLIPS) readily enhances RIPK1/RIPK3/MLKL-mediated necroptosis following treatment with TNF and IAP-antagonists (Geserick et al. 2009; Feoktistova et al. 2011, 2012). Under the same conditions, expression of the long isoform (cFLIPL) blocks necroptosis. Thus, while both cFLIP isoforms suppress apoptotic cell death, expression of cFLIPL, but not cFLIPS interferes with necroptotic cell death in the absence of cIAPs.
CELL LINES FOR STUDYING DIFFERENT CELL DEATH MODALITIES
To illustrate different modalities of cell death, we have used different cell lines: HT1080, L929, HaCaT, and HeLa cells (Feoktistova et al. 2011; Tenev et al. 2011). HaCaT cells (Boukamp et al. 1988) are primary keratinocyte-derived transformed cells that express low levels of cFLIP and XIAP, but that harbor high levels of cIAPs and caspases (Leverkus et al. 2000, 2003). HaCaT cells are highly sensitive to death receptor-induced cell death, and are thus a suitable model cell line to study molecular, biochemical, and morphological characteristics of the apoptotic and necroptotic signaling machinery. Cells of the human fibrosarcoma cell line HT1080 display classical apoptotic cell death features in response to DNA damaging agents or death receptor stimulation. HT1080 and HeLa cells do not express detectable levels of RIPK3 and hence die exclusively by apoptosis and are, therefore, useful to investigate cell death pathways independent of RIPK3-mediated necroptosis. To study necroptosis in HT1080 cells we have created a HT1080 cell line with inducible expression of RIPK3 (HT1080ind-RIPK3). In contrast to HT1080 cells, murine fibroblast-like L929 cells naturally contain high levels of endogenous RIPK3, and readily undergo necroptosis following treatment with TNF (Vercammen et al. 1998).
PROTOCOLS
In the accompanying protocols, we present a number of techniques for the analysis of apoptosis and necroptosis in cultured cells. See Protocol: Crystal Violet Assay for Determining Viability of Cultured Cells (Feoktistova et al. 2015a), Protocol: Analysis of Apoptosis and Necroptosis by Fluorescence-Activated Cell Sorting (Wallberg et al. 2015a), Protocol: Time-Lapse Imaging of Cell Death (Wallberg et al. 2015b), and Protocol: Ripoptosome Analysis by Caspase-8 Coimmunoprecipitation (Feoktistova et al. 2015b). These techniques enable the visualization and quantification of the effects on cell death of the tools and manipulations described above. Time-lapse video microscopy can be used to provide further insight into the morphological characteristics of dying cells. Biochemical studies that address the kinetics of the assembly of intracellular cell death platforms provide further insight into the molecular mechanisms of cell death. Additional and complementary techniques are discussed elsewhere (Krysko et al. 2008).
Footnotes
-
↵5 Correspondence: pascal.meier{at}icr.ac.uk; mleverkus{at}ukaachen.de
- © 2016 Cold Spring Harbor Laboratory Press