Assays to Measure Insecticide Toxicity and Insecticide Resistance in Mosquitoes
- Nannan Liu1,3,
- Yifan Wang1 and
- Ting Li1,2
- 1Department of Entomology and Plant Pathology, Auburn University, Auburn, Alabama 36849, USA
- 2Department of Biological Sciences, Alabama State University, Montgomery, Alabama 36104, USA
- ↵3Correspondence: liunann{at}auburn.edu
Abstract
Mosquitoes’ resistance to commonly used insecticides is now widespread, hampering control efforts and leading to substantial increases in human illness and mortality rates in many areas of the world. Insecticide bioassays are quantitative methodologies used to determine the dose–response relationship of insects to insecticides and to evaluate the susceptibility or resistance of mosquitoes to specific insecticides. They are frequently used to monitor the development of insecticide resistance in mosquitoes for both field resistance diagnoses (surveillance assays), in which the ability of mosquitoes to survive exposure to a standard dose or concentration of an insecticide is measured, and laboratory bioassays, in which responses to insecticides are tested in parallel populations of resistant (field) populations and laboratory susceptible strains using serial doses or concentrations. Metabolic detoxification, in which insecticides are metabolized by enzymes, including cytochrome P450s, hydrolases, and glutathione-S-transferases (GSTs), to become more polar and less toxic, is one resistance mechanism. Piperonyl butoxide (PBO), S,S,S-tributyl phosphorotrithioate (DEF), and diethyl maleate (DEM) are the inhibitors of P450s, hydrolases, and GSTs, respectively, and act as synergists for rapidly testing the involvement of these enzymes in insecticide resistance. Such synergistic assays are used to identify the detoxification enzyme that leads to resistance to a specific insecticide. This introduction and its associated protocols present a detailed discussion of appropriate methodologies and procedures for laboratory larval, adult, and synergistic bioassays and introduces the field surveillance tests used to monitor insecticide resistance as recommended by the latest World Health Organization (WHO) and U.S. Centers for Disease Control (CDC) guidelines.
MOSQUITO VECTORS AND INSECTICIDE RESISTANCE
Vector control of mosquitoes has long been a critical part of the current global strategy to control mosquito-associated diseases, with insecticides such as pyrethroids, carbamates, and organophosphates (Liu 2015; Choi et al. 2019; Sahu et al. 2020; World Health Organization 2020) being the most important component in these efforts. In the past, massive spraying of insecticides significantly limited outbreaks of mosquito-borne diseases and even eradicated malaria in a few areas (Hemingway et al. 2002). However, the widespread development of resistance to the most commonly used insecticides in mosquitoes is now hampering mosquito control efforts worldwide (Hemingway et al. 2002; Zaim and Guillet 2002; World Health Organization 2020), leading to substantial increases in human illness and mortality rates in many areas of the world (World Health Organization 2020). Research on insecticide resistance in mosquitoes has proliferated since the 1950s, when the first report of resistance to chlorinated-hydrocarbon insecticides in mosquitoes was published (Giullin and Peters 1952). Investigators worldwide are attempting to elucidate the toxicity of insecticides and the sensitivity of mosquitoes to the insecticides currently used to understand the development and status of insecticide resistance in field populations. Doing so is a vital first step toward the creation of new, more effective strategies that prevent resistance development, control resistant mosquitoes, and, ultimately, reduce the prevalence of mosquito-borne diseases.
THE USE OF INSECTICIDE ASSAYS TO MEASURE MOSQUITOES’ RESPONSE TO INSECTICIDES AND MONITOR THE DEVELOPMENT OF INSECTICIDE RESISTANCE
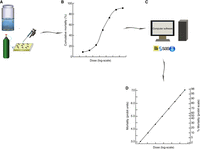
Insecticide assays are quantitative methodologies used for determining the relationship between the amount (i.e., dose or concentration) of an insecticide administered in insects and their subsequent response. This relationship, which is referred to as the dose–response relationship, is a fundamental feature of insecticide bioassays (Fig. 1) and the information revealed is critical for evaluating the susceptibility or resistance of mosquitoes to insecticides. The insecticide bioassays most frequently used in mosquito research include both insecticide surveillance/diagnosis assays, which examine the ability of mosquitoes to survive exposure to a standard dose or concentration of an insecticide, and laboratory bioassays, in which the responses to insecticides are tested in parallel resistant (field) populations and laboratory susceptible strains with a series of doses or concentrations across a range from 0% to 100% mortality (Liu et al. 2004).
Schematic diagram of quantal dose–response relationship of insects’ response to chemicals. (A) Insecticide bioassays for mosquito larvae and adults. (B) Dose–response curve with a cumulative mortality plotted along the y-axis and the logarithm of the dose or concentration being plotted along the x-axis. (C) Mathematical transformation of probit (probability unit) analysis using appropriate computer software. (D) Dose–response curve of mortality in probits with the logarithm of the dose or concentration being plotted along the x-axis and the percent mortalities, converted to probits, being plotted along the y-axis.
INSECTICIDE RESISTANCE SURVEILLANCE
Monitoring insecticide resistance in the field is critical for understanding the resistance status of field populations. To achieve this, a field population is exposed to a concentration of insecticide that reliably kills susceptible insects, so any survivors are presumed to be resistant. The World Health Organization's (WHO's) standard tube-test method and the U.S. Centers for Disease Control's (CDC's) bottle bioassays are commonly used for insecticide resistance surveillance tests. Both tests are designed to distinguish between baseline susceptibility and resistance to insecticides in adult mosquitoes by exposing the mosquitoes to known concentrations of an insecticide for a fixed period of time. The CDC bottle assay uses a bottle coated with a known amount of insecticide at the diagnostic concentration, which is the concentration of the insecticide known to kill 100% of susceptible mosquitoes within a given time (Centers for Disease Control and Prevention 2019). Resistance is assumed to be present if a significant portion of the test population survives the diagnostic concentration within the diagnostic time, with the level of resistance to that insecticide being determined based on the percentage of mosquitoes that die (mortality rate) before the cutoff time. In the WHO susceptibility assays, mosquitoes are loaded into a set of holding tubes containing paper coated with a “discriminating concentration” of an insecticide, which is the concentration of an insecticide known to kill 100% of susceptible mosquitoes in a given amount of time. Intensity assays test susceptibility of mosquitoes at 5× and 10× concentration of the discriminating concentrations of insecticides (World Health Organization 2016). The detailed diagnostic concentrations, threshold time, and standard determination of susceptibility/resistance for the majority of commonly used insecticides are listed in the guidelines issued by the World Health Organization (2016) and the Centers for Disease Control and Prevention (2019) for their respective resistance surveillance protocols.
INSECTICIDE LABORATORY BIOASSAYS
Insecticide bioassays of insects are often used to estimate the median lethal dose (LD50) or concentration (LC50) and/or the 90% lethal dose (LD90) or concentration (LC90) of an insecticide along with the associated 95% confidence intervals (95% CI), in dose–response models. The LD50 or LC50 is the dose or concentration of an insecticide required to kill 50% of a given population or strain under the specified conditions in a given time period. The 95% confidence interval signifies a range of values where the true mean of the population is 95% certain (Burgess et al. 2020). The LD50 (or LC50) and the 95% CI obtained for the dose–response relationship is generated via a probit analysis (Finney 1971; Raymond 1985), a method commonly used to analyze the relationship between a stimulus (dose) and the quantal response (Fig. 1). A probit analysis allows researchers to mathematically transform the sigmoidal normal distribution of dose–response data (in this case, the mortality of the insects) into a cumulative normal distribution for the response that can then be analyzed by performing a linear regression, with the logarithm of the dose or concentration being plotted along the x-axis and the percent mortalities, converted to probits (probability units), being plotted along the y-axis (Aldrich and Nelson 1984; Agresti 2015). Levels of insecticide resistance in insects are characterized by comparing the LD50 or LC50 measured for the resistant and susceptible strains (populations that have not been exposed to insecticides) (Liu et al. 2004). It has been suggested that an insect population should be referred to as resistant when the resistance ratio is as low as >4 (Scott and Wen 1997); others have argued that insects should not be considered resistant until a resistance ratio of 10 is reached (Valles et al. 1997). Here, we consider an insect population with a resistance ratio for a specific insecticide between 1 and 10 to be tolerant rather than resistant, and the resistance is defined when the resistance ratio is >10. (Liu et al. 2004).
Insecticide bioassays provide a reliable and informative methodology with which to measure the median lethal dose, lethal concentration, and toxicity of a specific insecticide for a specific insect population and are thus essential for the evaluation of levels of resistance of an insect population to an insecticide. Laboratory insecticide bioassays are frequently used by researchers to evaluate the susceptibility and resistance of mosquitoes to insecticides and are increasingly being used to generate comparative toxicity data for different chemicals and different populations, often within a short time period. This introduction and the associated protocols therefore discuss both adult and larval bioassays (see Protocol: Mosquito Larval Bioassays [Wang et al. 2023] and Protocol: Mosquito Adult Bioassays [Li et al. 2023a]).
SYNERGISM STUDY
Insecticide resistance in mosquitoes develops as a result of complex interactions among multiple resistance mechanisms or genes. Increased metabolic detoxification is one such mechanism in mosquitoes. The detoxification of insecticides in mosquitoes involves three major metabolic detoxification gene families: cytochrome P450s (P450s), hydrolases, and glutathione-S-transferases (GSTs), which can metabolize/detoxify the insecticides, making them more polar and less toxic. Piperonyl butoxide (PBO), S,S,S,-tributyl phosphorotrithioate (DEF), and diethyl maleate (DEM) are the inhibitors of P450s, hydrolases, and GSTs, respectively, and act as synergists for rapidly testing the involvement of these enzymes in insecticide resistance of mosquitoes. Using synergists in insecticide bioassays to examine the corresponding detoxification enzymes in the development of insecticide resistance is called a synergism study (see Protocol: Synergism Study for Investigating Possible Mechanisms of Insecticide Resistance in Mosquitoes [Li et al. 2023b]). In these studies, a synergist(s) is applied to mosquitoes together with an insecticide. For example, synergism studies of pyrethroid insecticide resistance in mosquitoes have enabled researchers to draw diagnostic conclusions regarding whether and which detoxification mechanisms are involved in the development of pyrethroid resistance (Kasai et al. 1998; Brogdon et al. 1999; Enayati et al. 2003; Liu et al. 2004; Xu et al. 2005). However, conclusions drawn from synergistic studies alone must be treated with caution; although using synergists may often correctly indicate the role of detoxification proteins in insecticide resistance, in some cases the synergists may be only imperfect inhibitors of some of the detoxification enzymes responsible for resistance (Feyereisen 2005). Further work is thus needed to support the findings from synergistic studies. The detailed methodology required for such studies is described in our associated protocol (see Protocol: Synergism Study for Investigating Possible Mechanisms of Insecticide Resistance in Mosquitoes [Li et al. 2023b]).
CONCLUSION
In this introduction and the associated protocols, we provide detailed methodologies for the most commonly used types of laboratory bioassays, including the larval bioassays and adult bioassays (topical and bottle residual) used to characterize the toxicity of insecticides and evaluate levels of insecticide resistance. We also introduce synergism assays used to test possible mechanisms of metabolic detoxification-mediated resistance conferred by important metabolic enzymes, especially P450s, GSTs and hydrolases. Synergism studies provide a useful way to measure the target proteins involved in the development of insecticide resistance and other proteins that may be involved in the regulation of insecticide resistance once the inhibitors of the target proteins are known. Each protocol provides a detailed methodology, describing how the assays should be conducted and ways of dealing with problems that may arise that could influence the accuracy of the data analyses and results. Finally, we introduce the field surveillance tests used to monitor insecticide resistance based on the protocols presented in the World Health Organization (2016) and Centers for Disease Control and Prevention (2019) recommendations.
Footnotes
-
From the Mosquitoes collection, edited by Laura B. Duvall and Benjamin J. Matthews.