Cover image
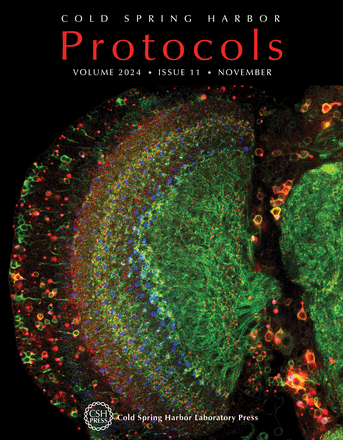
Sleep is a fundamental feature of life for virtually all multicellular animals, but many questions remain about its regulation by the circadian clock and other factors, and its biological functions. The fruit fly Drosophila melanogaster has been an important model system for the study of both circadian rhythms and sleep, and analysis of individual fly activity using Drosophila activity monitors (DAMs) has provided relevant insights into these processes. In this issue, Vecsey et al describe approaches for the activation of specific neurons of interest in Drosophila using optogenetics and thermogenetics to study the role of specific neurons in controlling rest and activity behavior, enabling the analysis of sleep and circadian rhythms in this organism (doi:10.1101/pdb.prot108180). The cover image shows the optic lobe in the central nervous system of a Drosophila adult fly expressing a synaptic label, Brp-Short (red), and a membrane label, mCD8-GFP (green), under the control of DIPγ-GAL4, in Dm8 neurons. The sample is costained with anti-Chaoptin (blue), which labels the R7 photoreceptor axons. R7 cells synapse onto Dm8 neurons, which then connect downstream to Tm5c neurons to forward visual information from the environment into the central brain through a series of connections. In the absence of visual information and light input from photoreceptors, circadian rhythms are disrupted in the adult fly, leading to alterations in the free-running clock. Image provided by Dr. Michael Aimino of the Mosca Laboratory at Thomas Jefferson University.



