Voltage-Clamp Analysis of Synaptic Transmission at the Drosophila Larval Neuromuscular Junction
- Bing Zhang1,3 and
- Bryan Stewart2,3
- 1Division of Biological Sciences, University of Missouri, Columbia, Missouri 65211, USA
- 2Department of Biology, University of Toronto at Mississauga, Mississauga, Ontario L5L 1C6, Canada
- ↵3Correspondence: zhangbing{at}umsystem.edu; goodfruitflies{at}gmail.com; bryan.stewart{at}utoronto.ca
Abstract
Although it is particularly valuable in revealing membrane potential changes, intracellular recording has a number of limitations. Primarily, it does not offer information on the kinetics of membrane currents associated with ion channels or synaptic receptors responsible for the potential change. Furthermore, the resting potential of the Drosophila body wall muscle varies naturally such that the driving force also varies considerably, making it difficult to accurately compare the amplitude of miniature synaptic potentials (minis) or evoked excitatory junction potentials (EJPs). Finally, accurate determination of quantal content based on minis and EJPs is possible only under low-release conditions when nonlinear summation is not a major issue. As the EJP amplitude increases, it creates a “ceiling effect,” because the same amount of transmitter will be less effective in depolarizing the membrane when the potential is approaching the reversal potential of glutamate receptors/channels. To overcome these limitations, the voltage-clamp technique can be used, which uses negative feedback mechanisms to keep the cell membrane potential steady at any reasonable set points. In voltage-clamp mode, the amplitude and kinetics of membrane currents can be determined. In the large larval muscle cells of Drosophila, the two-electrode voltage-clamp (TEVC) method is used, in which one electrode monitors the cell membrane potential while the other electrode passes electric currents. This protocol introduces the application of TEVC in analysis of synaptic currents using the larval neuromuscular junction preparation.
MATERIALS
Reagents
Drosophila melanogaster synaptic transmission mutants (e.g., Syx3-69, snap-25ts, syt I, and lap), neuronal excitability mutants (e.g., eag, Sh double mutant), or muscle excitability mutants (e.g., larvae that overexpress the inward rectifier potassium channel Kir2.1 in body wall muscles [referred to as the Kir “mutant”])
D. melanogaster wild-type strains (Canton-S or Oregon-R)
-
Maintain and raise all flies (mutants and wild type) on cornmeal-based fly food at room temperature. Avoid crowding in the vials, so that larvae can grow healthy and large.
HL-3 saline without Ca2+, ice-cold
KCl (3 m)
Equipment
Computer with pClampex software
Dissection instruments (Sylgard-coated Petri dish and small insect pins or a magnetic dish with paper clip pins, and fine scissors)
Electrophysiology rig including Axoclamp amplifier and A/D board (see Introduction: Synaptic Electrophysiology of the Drosophila Neuromuscular Junction [Zhang and Stewart 2024d])
Microelectrode, current-passing (as prepared in Protocol: Fabrication of Microelectrodes, Suction Electrodes, and Focal Electrodes for Electrophysiological Recording in Drosophila [Zhang and Stewart 2024a])
-
Prepare the current-passing electrode with an input resistance of 5–8 MΩ so that it will be easier to inject current into muscles.
Microelectrode, voltage-monitoring (as prepared in Protocol: Fabrication of Microelectrodes, Suction Electrodes, and Focal Electrodes for Electrophysiological Recording in Drosophila [Zhang and Stewart 2024a])
-
Prepare the voltage-monitoring electrode with an input resistance of 15–18 MΩ.
MicroFil needle
Microscope
-
When using an H2O-immersion lens (such as 60×) to visualize the neuromuscular junction (NMJ) for electrophysiology, a long working distance is required to provide room for positioning three electrodes (voltage, current, and suction).
-
For safety reasons, an “electrophysiology-ready” specialized H2O-immersion lens must be used. The tip of the lens is coated with electrical insulators, such as ceramics or special resins (e.g., LUMPLFL 60 W/IR objective from Olympus). Voltage-clamp methods generate high voltages through the current-passing electrode. A metal-coated lens dipped in saline will short-circuit the amplifier and could be hazardous to the user and the equipment.
Suction electrode (as prepared in Protocol: Fabrication of Microelectrodes, Suction Electrodes, and Focal Electrodes for Electrophysiological Recording in Drosophila [Zhang and Stewart 2024a])
Syringe (1-mL or 5-mL)
METHOD
-
This is a general protocol that will need to be adapted for the specific equipment being used.
-
See Protocol: Recording from Drosophila Larval Body Wall Muscles: Passive Membrane Properties and Basic Features of Synaptic Transmission (Zhang and Stewart 2024c) for additional background on this protocol.
-
1. Keep the head stage (HS-2A-x0.1 LU) and connections to the AD board for intracellular (bridge) mode (as described in Protocol: Electrophysiological Recording from a “Model” Cell [Zhang and Stewart 2024b]). Connect the current-passing and low-resistance head stage (e.g., HS-2A-x1LU) to the ME2 channel on the back of the amplifier.
-
Avoid touching the current injection head stage while it is in two-electrode voltage-clamp (TEVC) mode. Switch back to the bridge mode if the electrode needs to be changed.
-
-
2. Connect V2 and I2 outputs to defined input channels (such as channels 2 and 3, respectively) on the AD board and set up the pClampex software accordingly to record signals from these channels.
-
Use a model cell to practice TEVC if unfamiliar with the operation of the amplifier and the software used for voltage-clamp experiments (see Protocol: Electrophysiological Recording from a “Model” Cell [Zhang and Stewart 2024b]).
-
-
3. Pull voltage and current microelectrodes (as described in Protocol: Fabrication of Microelectrodes, Suction Electrodes, and Focal Electrodes for Electrophysiological Recording in Drosophila [Zhang and Stewart 2024a]), fill them with 3 m KCl using a syringe and MicroFil needle, and place them in the electrode holders.
-
4. Dissect and prepare a Drosophila larval body wall preparation in ice-cold Ca2+-free HL-3 saline as described in Introduction: Cell Biology Techniques for Studying Drosophila Peripheral Glial Cells (Clayworth et al. 2023a), Protocol: Dissection and Immunolabeling of the Central and Peripheral Nervous System of Drosophila Larvae (Clayworth et al. 2023b), and Protocol: Recording from Drosophila Larval Body Wall Muscles: Passive Membrane Properties and Basic Features of Synaptic Transmission (Zhang and Stewart 2024c).
Performing TEVC
-
5. To practice using TEVC, measure spontaneous miniature synaptic potentials (minis) without stimulating the nerve.
-
6. Remain in the “bridge mode.” Set the electrode potentials to 0, check the input resistance, and balance the bridge after the electrode is in the HL-3 bath solution.
-
7. Impale the target muscle fiber with the current-passing electrode. Measure the resting potential.
-
Because it has a larger tip (hence a lower resistance) than an intracellular electrode, the current-passing electrode can cause more damage to the muscle membrane and consequent deterioration in the initial resting potential.
-
See Troubleshooting.
-
-
8. Once the resting potential of the muscle has recovered, impale the same muscle fiber with the voltage electrode. Wait 1–2 min until the readings from both electrodes become similar.
-
If these readings differ by more than a few millivolts, or if the resting potential is poor, reject this muscle and try again with a new muscle fiber.
-
If the readings are similar, proceed immediately to Step 9.
-
-
9. Tune the clamp conditions to ensure that the muscle fiber can be clamped at a set voltage and that the quality of the current response is fast and not too noisy.
-
i. Set the clamp “gain” to the minimum and turn the “phase lag” and “anti-alias” filter off completely.
-
ii. Adjust the “holding position” dial until the “RMP balance” LEDs reach an equal intensity (which should be dim).
-
This allows the holding potential to be close to the resting potential of the muscle. If the holding potential is far from the resting potential, the muscle could be killed because of excessive current shock.
-
-
-
10. Switch from the “bridge” mode to “TEVC” mode by pressing the large blue TEVC button.
-
11. Set the holding potential to –80 mV. Set the step command to +20 mV.
-
12. Repeatedly deliver a 5-Hz square pulse (500 msec in duration) and observe the shape of the command voltage from the voltage electrode (the Vm channel), and the current (the I2 channel) and voltage responses (the V2 channel) from the current electrode.
-
The initial response will not look like a perfect square wave, and the peak amplitude of the command voltage may not reach 20 mV.
-
See Troubleshooting.
-
-
13. Gradually increase the gain to achieve the best square wave allowed under the experimental conditions.
-
The voltage trace should appear sharper, and its rise and fall times should be faster.
-
Avoid turning the gain too high, which will lead to oscillations and likely kill the clamped cell. The current trace will have two capacitance components (an initial and a tail current), which initially have a slower time course and should also become sharper and faster when the gain is properly set.
-
-
14. To increase further the speed of the voltage and current responses, careful apply capacitance neutralization of the voltage-monitoring electrode on ME1.
-
There is no need to adjust ME2.
-
Recording Miniature Synaptic Currents
-
15. Once the holding potential is steady and the noise level of the current trace is low, begin recording miniature synaptic currents. Alter the holding potentials from –80 mV to –100, –90, –60, and –40 mV, and observe the change in miniature synaptic current amplitude.
-
See Troubleshooting.
-
-
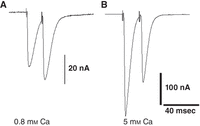
16. Practice until fully comfortable with TEVC, and then use the suction electrode to stimulate the motor nerve and record evoked synaptic currents (Fig. 1).
-
17. Vary the extracellular Ca2+ concentration, determine the evoked synaptic current amplitude at each concentration, and compare them at the same holding potential. Also, vary the holding potential and observe the change in evoked synaptic current amplitude at a given Ca2+ concentration.
-
See Troubleshooting.
-
-
18. Examine short-term synaptic plasticity by measuring twin-pulse facilitation or depression. Monitor long-term depletion of the synaptic vesicle pool by prolonged and repetitive stimulation. Use the same stimulation parameters that were used for excitatory junction potential (EJP) recordings in Protocol: Recording from Drosophila Larval Body Wall Muscles: Passive Membrane Properties and Basic Features of Synaptic Transmission (Zhang and Stewart 2024c).
Two-electrode voltage-clamp (TEVC) analysis of synaptic transmission at the larval neuromuscular junction (NMJ). Short-term synaptic facilitation (A) and depression (B) at a wild-type larval NMJ are revealed by TEVC recording of synaptic currents at different [Ca2+] in response to twin-pulse stimuli (50 Hz).
TROUBLESHOOTING
Problem (Step 7): After inserting the current-passing electrode, the muscle resting potential was very poor (–20 mV) and never recovered to the normal range.
Solution: The large-tipped current-passing electrode can easily injure muscle cells. The poor resting potential results from an unregulated influx of Na+ ions into the cell through the damaged membrane. Consider fabricating sharper and more efficient current-passing electrodes. Bevel the tip of the electrode on a grinder at a 45° angle to sharpen the bottom portion of the tip. This creates a more pointed tip with a larger opening. In addition, the muscle will be better “space-clamped” because of the increased efficiency of current passing.
Problem (Step 12): Both electrodes recorded very good resting potentials, but the voltage electrode did not respond at all to current injection via the current-passing electrode.
Solution: The body wall muscles have three layers. It is likely that the two electrodes were not in the same muscle fiber. One of the electrodes likely penetrated a muscle fiber below the target muscle. Replace the current-passing electrode and try again.
Problem (Step 15): The baseline of the clamped current was not steady.
Solution: Small variations of the baseline over time are expected. However, large variations indicate poor “space clamp.” Start with another muscle with new electrodes.
Problem (Step 15): The TEVC currents are too noisy.
Solution: Reduce noise using common methods, such as shielding, common grounding, etc. In addition, lower bath saline levels. Keep the recording chamber dry to reduce noise.
Problem (Step 17): Muscle contraction disrupted what was a perfect clamp.
Solution: Losing a good clamp to muscle contraction, especially when the nerve is stimulated at a high frequency at high Ca2+ concentrations, is expected. Two things can be done that will reduce (although not eliminate) the chance of muscle contractions for TEVC and for all other recordings. When pinning the larvae, try to stretch them out as much as possible, without tearing the muscle or the preparation. Also, perform the experiments in a cooler room (18°C–20°C) or on a cooled microscope stage to reduce the chance of muscle contractions.
ACKNOWLEDGMENTS
We thank the members of our laboratories for their important contributions to our research programs. B.Z. especially thanks Dr. Hong Bao for her expert assistance with the Neurobiology of Drosophila course and for providing some of the figures. Work in the Zhang laboratory was supported by grants from the National Science Foundation, the National Institutes of Health, the Oklahoma Center for the Advancement of Science and Technology, and research funds from the University of Oklahoma and the University of Missouri. B.Z. also thanks Dr. Carlos Rivera, Dr. Salleh Ehaideb, Dr. Phillip Vanlandingham, Dr. Rudhof Bohm, and Dr. Hong Bao for their constructive comments. Work in B.S.’s laboratory was supported by a Canada Research Chair and grants from the Canadian Institute for Health Research, the Natural Science and Engineering Research Council of Canada, and the Canadian Foundation for Innovation.
Footnotes
-
From the Drosophila Neurobiology collection, edited by Bing Zhang, Ellie Heckscher, Alex C. Keene, and Scott Waddell.