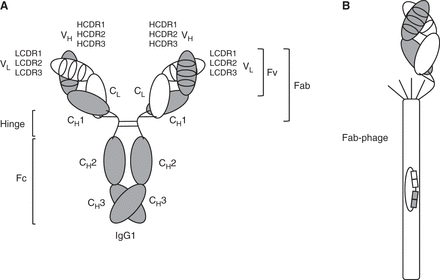
IgG1, Fab, and Fab-phage. (A) Depiction of an ∼150-kDa IgG1 molecule with the two ∼25-kDa light chains shown in white and the two ∼50-kDa heavy chains shown in gray. Each of these chains (light and heavy) comprises an N-terminal variable domain (VL or VH, respectively), each having three complementarity determining regions (CDRs), followed by one constant domain in the light chain (CL) and three constant domains in the heavy chain (CH1, CH2, and CH3). VL/VH and CL/CH1 heterodimerize, and CH3/CH3 homodimerizes. The glycosylated CH2 domain does not dimerize. The hinge region between CH1 and CH2 includes four interchain disulfide bridges: two between the heavy chains and two between heavy and light chains. The VL/VH heterodimer is also known as “fragment variable” (Fv), and the combined VL/VH and CL/CH1 heterodimer is known as the “fragment antigen binding” (Fab). The hinge region connects the two Fab arms of the IgG1 molecule to the “fragment crystallizable” (Fc). (B) Depiction of a Fab-displaying filamentous phage in phagemid-based phage display systems. Note the preserved C-terminal disulfide bridge between the light chain and heavy chain fragments. The Fab is fused to the N terminus of a C-terminal fragment of minor coat protein pIII and forms, together with four wild-type pIII copies, the spiky cap of the filamentous phage particle. The phagemid encapsulated by the filamentous phage particle encodes the displayed Fab, affording the physical linkage of genotype and phenotype.










