Imaging Gene Expression in Live Cells and Tissues
- Hao Hong,
- Yunan Yang and
- Weibo Cai
INTRODUCTION
Monitoring gene expression is crucial for studying the responses of gene therapy and clarifying gene function in various environments. Molecular imaging is a powerful tool for noninvasive visualization of gene expression. This article summarizes the current status of fluorescence and bioluminescence imaging (BLI) of gene expression in live cells and tissues, with the emphasis mainly on the early studies that pioneered the field. First, we describe fluorescence imaging of gene expression with a wide variety of fluorescent proteins. Next, we discuss the strategies for BLI of gene expression. Besides incorporating the reporter gene into the host DNA, mRNA-based BLI of gene expression is also briefly mentioned. Last, the construction of double- and triple-fusion reporter genes is presented. Because no single imaging modality is perfect and sufficient to obtain all of the necessary information for a given question, combinations of multiple molecular imaging modalities can offer synergistic advantages over any modality alone. Noninvasive optical imaging of gene expression has revolutionized biomedical research, and the progress made over the last decade should allow molecular imaging to play a major role in the field of gene therapy. For basic and preclinical research, optical imaging is indispensable for imaging gene expression. However, for clinical imaging of gene expression, positron emission tomography (PET) holds the greatest promise.
OVERVIEW
With the ability to engineer genes and to create knockin and knockout models of human diseases, it has become clear that gene therapy can play an important role in disease management. Over the last four decades, gene therapy has moved from preclinical to clinical studies for diseases ranging from monogenic recessive disorders (e.g., hemophilia) to more complex diseases, such as cancer, cardiovascular disorders, and human immunodeficiency virus (HIV) infection (Serganova et al. 2008; Gillet et al. 2009). To choose the appropriate gene therapy strategy and to optimize the therapeutic efficacy, an effective monitoring system is needed. One of the most economical and practical approaches is to adopt certain molecular imaging techniques for monitoring gene expression in vivo.
In recent years, noninvasive molecular imaging has emerged as a powerful tool for monitoring cellular and molecular events in vivo (Cai and Chen 2007; Cai et al. 2008a,b). A variety of imaging technologies is being investigated as tools for evaluating gene therapy efficiency and for studying gene expression in living subjects. Noninvasive, longitudinal, and quantitative imaging of gene expression can help in human gene therapy trials and can also facilitate preclinical experimental studies in animal models. Radionuclide-based imaging techniques (i.e., single-photon emission computed tomography [SPECT] and PET) have the greatest clinical potential and offer many advantages for noninvasive imaging of gene expression.
Besides imaging gene vectors to indirectly visualize the gene expression efficiency, PET and SPECT can play a significant role in imaging gene expression using diverse reporter genes and reporter probes (Kang and Chung 2008). If transcription of a reporter gene is induced, translation of the reporter gene mRNA will lead to a protein product that can interact with the imaging reporter probe (administered in trace amounts for PET/SPECT applications; hence the term “tracer”). This interaction may be based on the intracellular enzymatic conversion of the reporter probe with the retention of the metabolite(s), or a receptor–ligand-based interaction.
Examples of intracellular reporters include the herpes simplex virus type 1 thymidine kinase (HSV1-tk) and its mutant gene (HSV1-sr39tk) (Gambhir et al. 1999, 2000; Najjar et al. 2009). Substrates that have been studied to date, as PET reporter probes for HSV1-tk, can be classified into two main categories: pyrimidine nucleoside derivatives (e.g., 2′-fluoro-2′-deoxy-5′-[124I]iodo-1β-D-arabinofuranosyluracil [124I-FIAU]) and acycloguanosine derivatives (e.g., 9-[4-[18F]fluoro-3-(hydroxymethyl)butyl]guanine [18F-FHBG]). A few examples of reporters on the surface of cells include the dopamine 2 receptor (D2R), receptors for the human type 2 somatostatin receptor (hSSTr2), and the sodium iodide symporter (NIS).
Although magnetic resonance imaging (MRI) has relatively low sensitivity, it can be used to image gene expression in vivo (Gilad et al. 2007, 2008). MRI has been used for imaging iron-related gene products (e.g., transferrin), certain enzymes (e.g., β-galactosidase [β-gal]), and chemical exchange saturation transfer-related products (e.g., polylysine-containing proteins) (Gilad et al. 2008).
Whereas PET/SPECT and MRI offer greater clinical potential than many other imaging modalities, optical imaging techniques, such as fluorescence and bioluminescence, have the following advantages: They are more economical, are easier to handle, do not need any radioisotopes, and are quite sensitive in certain scenarios. Therefore, optical imaging has been widely used for monitoring gene expression in live cells and tissues. In the remainder of this article, we summarize the current state of the art in optical imaging of gene expression.
FLUORESCENCE IMAGING OF GENE EXPRESSION
For fluorescence imaging of gene expression, green fluorescent protein (GFP; emission peak at 509 nm) and its variants are the mainstay among fluorescent probes (Fig. 1). Because light at longer wavelengths (i.e., red or near infrared) penetrates tissues better than light at shorter wavelengths, much effort has been devoted to engineering fluorescent proteins that can emit in the red or near infrared.
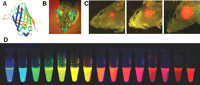
Fluorescence imaging of gene expression. (A) The structure of GFP. (B) A transgenic GFP mouse. (C) Real-time whole-body imaging of a GFP-expressing human glioma growing in the brain of a nude mouse at 1 wk (left), 3 wk (center), and 5 wk (right) after surgical orthotopic implantation. (D) Monomeric and tandem dimeric fluorescent proteins derived from Aequorea GFP or Discosoma RFP, expressed in bacteria, and purified. (Adapted, from Shaner et al. 2004; Hoffman and Yang 2006 and reprinted with permission from Macmillan © 2004, 2006.)
GFP-Based Imaging
From the early 1990s, GFP and its variants have become invaluable markers for monitoring protein localization and gene expression in vivo (Heim and Tsien 1996; Jakobs et al. 2000). One early example of imaging gene expression with GFP was the demonstration of vascular endothelial growth factor (VEGF) promoter activity (Fukumura et al. 1998). The VEGF promoter was linked to the GFP gene, and the expression of GFP was imaged by intravital microscopy. For such intravital studies, in which only limited tissue penetration of the reporter protein is needed, GFP was found to be readily detectable, confined to the cell, and stable (with a half-life of up to 24 h in cells). Although GFP penetrates tissue poorly, its expression can be visualized noninvasively in intact animals (Fig. 1; Yang et al. 2000; Hoffman and Yang 2006). GFP has also been used frequently in stem cell research to monitor gene expression (Meyer et al. 2000; Niyibizi et al. 2004). Modifications of GFP to increase its signal intensity and thermostability, as well as to alter its emission spectrum, have expanded the utility of GFP in gene transfer studies (Welsh and Kay 1997).
GFP is very useful for imaging tumor-related gene expression. Mouse models of metastatic cancer were developed with genetically fluorescent tumors, in which the GFP gene was cloned into cancer cell lines and selected for stable GFP expression, which was imaged in fresh tissue, both in situ and externally (Hoffman 2001). With this model system, tumor location and metastasis were detected and were visualized in situ within host organs down to the single-cell level. Imaging GFP-transfected tumor cells was a fundamental advance in visualizing tumor growth and metastasis in real time in vivo. In addition, tumor growth and metastatic development and inhibition of these processes by certain drugs could now be imaged and quantified for rapid screening of anticancer drugs. Research about imaging tumor-related gene expression has flourished, including the use of a reporter gene system consisting of enhanced green fluorescent protein (eGFP) and wild-type HSV1-tk for both fluorescence and PET imaging (Luker et al. 2002).
GFP can also serve as a powerful tool for imaging other diseases, such as HIV infection. For example, the function of the HIV-1 Tat protein (capable of traversing cell membranes) has been elucidated by fluorescence imaging (Ferrari et al. 2003). After exogenously adding Tat-GFP fusion protein to live HeLa and CHO cells, it was found that the internalization process of full-length Tat, as well as of heterologous proteins fused to the transduction domain of Tat, exploits a caveolar-mediated pathway. With fluorescence imaging, the dynamic movement of individual GFP-tagged, Tat-filled caveolae toward the nucleus was observed directly.
Red Fluorescent Protein-Based Imaging
In 1999, a new red fluorescent protein (RFP) was isolated from tropical corals and was termed DsRed (Matz et al. 1999). With emission maxima at 509 and 583 nm, respectively, eGFP and DsRed are well suited for virtually crossover-free, dual-color imaging upon simultaneous excitation. Mixed populations of Escherichia coli expressing either eGFP or DsRed were imaged by one-photon and two-photon microscopy. Both excitation modes were found to be suitable for imaging cells expressing either of the fluorescent proteins.
The predominant use of DsRed has been for multicolor imaging in plants, together with the GFP variants, because its red-shifted excitation/emission spectra avoid damaging plant cells and tissues by the excitation light (Heikal et al. 2000; Dietrich and Maiss 2002). Dual gene expression has also been imaged in animal models with GFP and DsRed-2 after transduction with a dual-promoter lentiviral vector (Chen et al. 2004). RFP-based gene expression imaging has also been used extensively in stem cell research. For example, the whole-body biodistribution and persistence of multipotent adult progenitor cells, transfected with DsRed-2, were visualized in vivo and in tissue sections (Tolar et al. 2005). Another important field for RFP-based research is cancer cell imaging. For example, fluorescent pancreatic cancer cells expressing a high level of the DsRed-2 gene were used to establish an orthotopic metastatic pancreatic cancer model (Zhou et al. 2008). The high-level expression of DsRed-2 enabled noninvasive imaging of distant micrometastases in their target organs, even in deep tissue such as the lung.
Wild-type DsRed has several drawbacks including inefficient folding of the protein, extremely slow maturation of the chromophore, and tetramerization even in dilute solutions. Therefore, stepwise evolution of DsRed to a dimer and then to a true monomer (designated as mRFP1) was performed (Campbell et al. 2002). The monomer and DsRed show similar brightness in living cells. In addition, the excitation and emission peaks of mRFP1, 584 and 607 nm, respectively, are ~25-nm red-shifted from DsRed, conferring greater tissue penetration and spectral separation from autofluorescence and other fluorescent proteins. Subsequently, a series of monomeric fluorescent proteins has been reported, which further expanded the toolbox for fluorescence imaging of gene expression (Fig. 1; Shaner et al. 2004; Wang et al. 2004).
Infrared Fluorescent Protein (IFP)
Generally speaking, GFP remains the first choice for fluorescence imaging of gene expression because it is relatively easy to fuse it with other genes and it has enhanced fluorescence characteristics compared with RFP. However, for optimal performance in in vivo imaging, near-infrared fluorescent (NIRF; 700–900 nm) proteins are superior. The NIRF signal can pass through deeper tissues, and the background fluorescence in this part of the spectrum is very low.
Recently, it was reported that a bacteriophytochrome from Deinococcus radiodurans, incorporating biliverdin as the chromophore, could be engineered into a monomeric IFP with excitation and emission maxima of 684 and 708 nm, respectively (Shu et al. 2009). The IFPs express well in mammalian cells and mice and spontaneously incorporate biliverdin, which is ubiquitous as the initial intermediate in heme catabolism but has negligible fluorescence by itself. These IFPs provided the basis for further engineering of IFPs with better performance characteristics, which is expected to find broad use in future biomedical research, in particular, those related to gene expression imaging.
β-Galactosidase-Based Imaging
Besides the fusion of fluorescent proteins with target gene products, several other strategies can also be adopted for fluorescence imaging of gene expression. The use of fluorogenic substrates for certain gene translation products (e.g., enzymes and proteins) is one choice. For example, the bacterial lacZ gene, which encodes β-gal, is a common reporter gene used in transgenic mice (Watson et al. 2008). However, the absence of fluorogenic substrates usable in live animals greatly hampered the applications of this reporter gene. In 2007, a far-red fluorescent substrate, 9H-(1,3-dichloro-9,9-dimethylacridin-2-one-7-yl) β-D-galactopyranoside, was developed for imaging β-gal expression (Josserand et al. 2007). With β-gal as a reporter of tumor growth, as few as 1000 β-gal-expressing tumor cells located under the skin could be detected with this substrate.
Tomographic Imaging of Gene Expression
Aside from the various strategies for imaging gene expression in live cells and tissues by fluorescence, as mentioned above, there have also been significant advances in imaging instrumentation. To generate a more quantitative fluorescence readout, fluorescence diffuse tomography has been developed recently for small animal imaging (Turchin et al. 2008). The animal is scanned in the transilluminative configuration by a single source and a detector pair, and a reconstruction algorithm was developed to estimate the fluorophore distribution. Another technique, fluorescence-mediated tomography (FMT), can also provide important insights into in vivo gene expression within deep tissues (Ntziachristos et al. 2003).
With the wide variety of tools for fluorescence imaging of gene expression, multiplexing is highly desirable and certainly possible. Using an imaging system capable of spectral unmixing, in which fluorophores with different emission spectra can be readily separated and analyzed simultaneously within the same sample (Cai et al. 2006; Cai and Chen 2008), multiple genes fused to different fluorescent proteins can be imaged at the same time, shedding new light into the biology and the mechanisms of various diseases. With newly developed fluorescent proteins that emit at longer wavelengths and the constantly evolving imaging systems, which can be used clinically to image certain sites of the human body (e.g., tissues close to the skin, accessible by endoscopy, and/or during surgery), fluorescence imaging of gene expression serves as an invaluable research tool for cell- and animal-based studies, as well as for certain potential clinical applications.
BIOLUMINESCENCE IMAGING OF GENE EXPRESSION
Another optical imaging technique, BLI, is an attractive alternative to fluorescence for optical imaging of gene expression. Although BLI has little, if any, clinical potential, it is virtually free of any background signal, an ideal property for sensitive imaging of gene expression in cells and small animals. Bioluminescence is the emission of light from biochemical reactions that occur within a living organism. The luciferases are a family of photoproteins that can be isolated from a variety of insects, marine organisms, and prokaryotes (Hastings 1996). Luciferase has been used as a reporter gene in transgenic mice, but, until the instrumentation was created for BLI, the detection of luciferase activity required either sectioning the animal or excising the tissue and homogenizing it to measure the luciferase activity in a luminometer (Sadikot and Blackwell 2008). BLI has proven to be a very powerful method for detecting luciferase activity in intact animal models. It is noninvasive, convenient, and relatively inexpensive, thus making it an excellent method for elucidating the pathobiology of diseases such as inflammation/injury, infection, and cancer in animal models. To date, firefly luciferase (Fluc) (Fig. 2A) and Renilla luciferase (Rluc) (Fig. 2B) are the most widely used reporter proteins for BLI of gene expression in living animals. Another type of luciferase, the bacterial luciferase, is primarily used in the study of infection because it is limited to bacteria that express the reporter gene and produce the substrate (Contag et al. 1995).
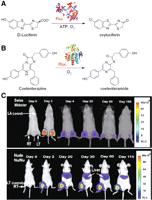
Bioluminescence imaging of gene expression. (A) The bioluminescent reaction catalyzed by Fluc. (B) The bioluminescent reaction catalyzed by Rluc. (C) The magnitude and the duration of Fluc gene expression in different host immune systems can be monitored noninvasively and repetitively using a cooled CCD camera. For Swiss Webster mice, the Fluc activity was highest at day 2 and significantly dropped over time. For nude mice, a considerable amount of Fluc activity was seen throughout the study period. (Adapted from Wu et al. 2001 and reprinted with permission from Macmillan © 2001.)
Fluc-Based BLI
Light emission (yellow–green color, 557 nm) from the firefly Photinus pyralis, which is generally believed to be the most efficient bioluminescence system known to date, makes Fluc an excellent tool for monitoring gene expression (Branchini et al. 2005). Fluc has been used to image gene expression in cells since the early 1990s (Hooper et al. 1990). In 2001, a method was described for repetitively tracking in vivo gene expression of Fluc in skeletal muscles of mice with a cooled charge-coupled device (CCD) camera (Fig. 2C; Wu et al. 2001). The in vivo bioluminescence signals correlated well with results from in vitro luciferase enzyme assays, which showed the ability of BLI to sensitively and noninvasively track the location, the magnitude, and the persistence of Fluc-related gene expression.
Fluc has broad applications in monitoring disease-related gene expression. For example, the cyclooxygenase-2 (COX-2) gene plays a role in a wide variety of physiologic pathways and is a major target for therapeutic intervention in many pathophysiologic contexts such as pain, fever, inflammation, and cancer. Expression of the COX-2 gene can be induced in a wide range of cells, in response to an ever-increasing number of stimuli. BLI was successfully performed to image the expression of the Fluc gene in tumor xenografts, which were stably transfected with a chimeric gene containing the first kilobase of the murine COX-2 promoter (Nguyen et al. 2003). The imaging data suggested that gene expression from the COX-2 promoter can be easily analyzed in a variety of disease models in which the COX-2 gene is up-regulated. BLI of gene expression with Fluc was also tested in larger animals such as rabbits (Li et al. 2005). It was reported that the BLI signal was capable of passing through at least 1 cm of muscle tissue.
BLI with Fluc is also applicable for the evaluation of DNA vaccines. Administration of naked DNA into animals has been used as a research tool for developing DNA vaccines. To monitor the distribution and the duration of gene expression of a DNA vaccine in living subjects, the naked DNA encoding Fluc was used as an imaging reporter gene in a mouse model (Jeon et al. 2006). It was concluded that BLI with Fluc could be useful for monitoring the location, the intensity, and the duration of gene expression of naked DNA vaccines in living animals, both noninvasively and repetitively. Similar to the study of DNA vaccines, BLI with Fluc (which serves as a model gene) can also be used to evaluate the gene delivery efficiency of certain gene delivery systems, especially in tumor models (Hildebrandt et al. 2003; Liang et al. 2004).
One major limitation of Fluc is that the light generated is in the yellow–green range, which has poor tissue penetration. In 2005, a set of red- and green-emitting luciferase mutants was reported (Branchini et al. 2005). The bioluminescence properties of these mutants are suitable for expanding the use of the P. pyralis system in dual-color reporter assays, biosensor measurements with internal controls, and imaging. Using a combination of mutagenesis methods, a red-emitting luciferase with a bioluminescence maximum of 615 nm was created, which also has a narrow emission bandwidth and favorable kinetic properties. Studies in animal models showed that these luciferases could detect gene expression at the attomole level, many orders of magnitude more sensitive than Fluc.
Rluc-Based BLI
Rluc is another promising bioluminescence reporter. Distinct from Fluc in terms of its origin, enzyme structure, and substrate requirements (Inouye and Shimomura 1997), Rluc was isolated from the sea pansy (Renilla reniformis), which displays blue–green bioluminescence upon stimulation. It can catalyze the oxidation of coelenterazine, which leads to a bioluminescence signal (Fig. 2B). Rluc was cloned and sequenced in 1991 (Lorenz et al. 1991) and has been used as a marker for gene expression in bacteria, yeast, plant, and mammalian cells (Lorenz et al. 1996).
BLI of gene expression with Rluc dates back to the early 2000s. In one pioneering report, Rluc was used for noninvasive BLI of advanced human prostate cancer lesions in living mice by a targeted gene transfer vector (Adams et al. 2002). In another early study, the ability to image gene expression based on Rluc bioluminescence was validated by injecting the substrate coelenterazine into living mice (Bhaumik and Gambhir 2002). Cells transiently expressing the Rluc gene, located in the peritoneum, subcutaneous layer, as well as in the liver and the lungs of living mice, were imaged after tail-vein injection of coelenterazine. Importantly, both Rluc and Fluc expression can be imaged in the same living mouse, although the kinetics of light production are distinct for the two enzymes. The imaging strategy validated in this study has direct application in various studies in which two molecular events need to be tracked, such as the trafficking of two cell populations, two gene therapy vectors, or indirect monitoring of two endogenous genes.
In a follow-up study, the expression in live mice of a novel synthetic Rluc reporter gene (hRluc) was explored; it has previously been reported to be a more sensitive reporter in mammalian cells than the native Rluc (Bhaumik et al. 2004a). It was found that hRluc:coelenterazine yielded stronger signals than the Fluc:D-luciferin in both cell culture and live animal studies.
Regarding the prognosis of cancer treatment, early detection of the tumor and its metastases is crucial. In animal models, Rluc can be used for detecting the expression of diagnostic genes in different stages of tumor development, thus delineating the location of primary and metastatic tumors (Yu et al. 2003). To achieve noninvasive measurement of chemotherapy-induced changes in the expression of genes related to tumor growth, BLI was used to image the alteration in human telomerase reverse transcriptase (hTERT) gene expression in tumor cells before and after 5-fluorouracil treatment (Padmanabhan et al. 2006). Several fusion reporters directed by the hTERT promoter fragments were investigated, which integrate the hRluc (for BLI), the mRFP1 (for fluorescence imaging), and a truncated thymidine kinase (for PET imaging with radiolabeled acycloguanosines), respectively. In vitro studies showed that although all three of the reporter systems can visualize the hTERT promoter activity, BLI was the most powerful one, and it could also provide assistance in choosing the optimal tracers for PET imaging (Wang et al. 2006).
BLI has been widely adopted for stem-cell-based studies. The discovery of human embryonic stem cells (hESCs) has dramatically expanded the tools available to scientists and clinicians in the field of regenerative medicine (Thomson et al. 1998). However, direct injection of hESCs, and cells differentiated from hESCs, into living organisms was hampered by significant cell death, teratoma formation, and host immune rejection. Understanding the hESC behavior in vivo after transplantation requires noninvasive imaging techniques, such as BLI, to longitudinally monitor the hESC localization, proliferation, and viability (Wilson et al. 2008). Bioluminescence reporter genes can be transcribed either constitutively or under specific biological or cellular conditions, depending on the type of promoter used. Stably transduced cells that carry the reporter construct within their chromosomal DNA will pass the reporter construct DNA to daughter cells, allowing for longitudinal monitoring of hESC survival and proliferation in vivo with BLI. Because expression of the reporter gene product is required for signal generation, only viable parent and daughter cells will generate a BLI signal, whereas the apoptotic or dead cells cannot, which is the key advantage of reporter gene-based cell tracking over direct cell labeling (Zhang and Wu 2007).
BLI of mRNA
In addition to incorporating Fluc or Rluc into DNA, a strategy for imaging mRNA with BLI was designed using spliceosome-mediated RNA trans-splicing (SMaRT) (Bhaumik et al. 2004b; Walls et al. 2008). SMaRT provides an effective means for reprogramming mRNAs and the proteins they encode. It can have a broad range of applications, including RNA repair and molecular imaging, each governed by the nature of the sequences delivered by the pre-trans-splicing molecule (PTM). In one groundbreaking study, the ability of SMaRT to optically image the expression of an exogenous gene at the level of pre-mRNA splicing was shown in both cells and living animals (Bhaumik et al. 2004b). Because of the modular design of PTMs, there is potential for SMaRT to be used for imaging the expression of any arbitrary gene of interest in living subjects.
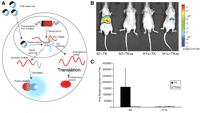
Recently, this strategy was improved (Fig. 3A; Walls et al. 2008) by using signal amplification and a facile method of delivery for developing a class of generalized probes capable of imaging pre-mRNA in a sequence-specific manner. Incorporating a modular binding domain that confers specificity by base-pair complementarity to the target pre-mRNA, the PTMs were designed to target a chimeric target minigene and trans-splice the Rluc gene onto the end of the target. After hydrodynamic delivery of the PTMs and target genes in mice, the efficiency and the specificity of the trans-splicing reaction were found to vary depending on the binding domain length and the structure (Fig. 3). Nonetheless, specific trans-splicing was observed in living animals that showed a proof of principle for a generalized imaging probe against RNA, which can amplify the signal upon detection and can be delivered with existing gene delivery methodology.
BLI of mRNA. (A) A schematic of the SMaRT imaging strategy. (B) Representative images of nude mice injected hydrodynamically with a combination of PTM and target plasmids, S2 + TK:PTM, and the target gene. The remaining three mice are negative controls. (C) Average radiance for each mouse (mean ± SEM) in photons/sec per cm2/steradian. (Adapted from Walls et al. 2008 and reprinted with permission from Society of Nuclear Medicine © 2008.)
BLI of gene expression has been used in a wide variety of disease models over the last decade, and the above-mentioned studies serve only as representative examples. Theoretically, Fluc or Rluc can be fused to almost any gene of interest, and the expression of the gene can be noninvasively monitored with BLI. One major advantage of BLI is that it is quite quantitative. Therefore, the changes in the bioluminescence signal can reflect the differences in gene expression level, provided that all other related variables remain constant (e.g., the depth of the tissue). Over the years, BLI has been used in a variety of model systems for applications ranging from stem cell trafficking to cancer therapy to drug screening. Bear in mind, however, that BLI is exclusively a research tool in preclinical studies and cannot be used in humans.
MULTIMODALITY IMAGING OF GENE EXPRESSION
Among all of the molecular imaging modalities, no single modality is sufficient to obtain all of the necessary information for a given question. For example, it is difficult to accurately quantify fluorescence signals in living subjects with fluorescence imaging alone, particularly in deep tissues; BLI is quantitative but is not suitable for clinical studies; and radionuclide-based imaging techniques, such as PET, are very sensitive but have relatively poor spatial resolution. A combination of multiple molecular imaging modalities can offer synergistic advantages over any modality alone. Various double-fusion and triple-fusion reporter genes have been reported for multimodality imaging of gene expression. In one early study, a reporter vector encoding a mutant HSV1-sr39tk and Rluc was constructed (Ray et al. 2003). The two genes were joined by a 20-amino-acid spacer sequence. Both PET and BLI were able to delineate tumors stably expressing the fusion gene in live xenograft-bearing mice. Subsequently, several triple-fusion reporter genes that can monitor bioluminescence, fluorescence, and PET imaging have been constructed. A triple-fusion reporter vector consisting of Rluc, RFP, and HSV1-sr39tk (ttk) was found to confer activity of every protein composition in cell culture (Ray et al. 2004). In xenograft mouse models, the lentiviral vector encoding the triple-fusion reporter gene also showed a good correlation of signals from different imaging modalities. Furthermore, the vector expression lasted for 40–50 d in live mice.
To exploit the combined strengths of each imaging technique and to facilitate multimodality imaging, a dual-reporter construct was established in which Fluc was fused in-frame to the N terminus of a mutant HSV-tk kinetically enhanced for PET (Kesarwala et al. 2006). In addition, a triple-reporter construct was developed in which monster GFP was introduced into the fusion vector downstream from an internal ribosome entry site to allow analysis by fluorescence microscopy or by flow cytometry without compromising the specific activities of the upstream fusion components. In mice, somatic gene transfer of a ubiquitin promoter-driven triple-fusion plasmid showed a >1000-fold increase in liver photon flux and a more than twofold increase in liver retention of 18F-FHBG by micro-PET when compared with mice treated with a control plasmid.
Construction and validation of several triple-fusion genes—each composed of a bioluminescent, a fluorescent, and a PET reporter gene in cell culture and in living subjects—were further explored (Ray et al. 2007). A mutant of a thermostable Fluc, bearing the peroxisome localization signal, was designed to have greater cytoplasmic localization and improved access for its substrate, D-luciferin. This mutant showed severalfold higher activity than Fluc both in vitro and in vivo. The improved version of the triple-fusion vector showed a significantly higher bioluminescence signal than the previous triple-fusion vectors. As the third reporter component of this triple-fusion vector for PET applications, a truncated version of wild-type HSV1-tk also retained a higher expression level than the truncated mutant HSV1-sr39tk. It was suggested that this improved triple-fusion reporter vector should enable high-sensitivity detection of lower numbers of cells in living animals using the combined bioluminescence, fluorescence, and PET imaging techniques.
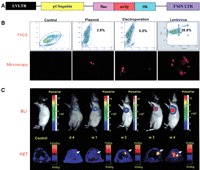
A proof-of-principle study for lentivirus-transduced murine embryonic stem cells (mESCs) that stably express this triple-fusion reporter gene was performed (Cao et al. 2006). It was shown that this molecular imaging platform can be used to monitor the kinetics of stem cell survival, proliferation, migration, and ablation of teratoma sites. The fluorescence feature of the reporter gene was used for cell sorting and microscopy studies, whereas both BLI and PET were used for longitudinal noninvasive monitoring of the transplanted mESCs (Fig. 4). Further development of novel imaging techniques will contribute important insights into the biology and the physiology of transplanted stem cells, leading to significant potential clinical applications for years to come.
Multimodality imaging of mESC survival, proliferation, and migration after cardiac delivery with a triple-fusion reporter gene. (A) Schema of the triple-fusion reporter gene. (B) Fluorescence-activated cell sorting (FACS) histograms of mESCs at 48 h after transduction with plasmid lipofectamine, electroporation, and lentivirus carrying the triple-fusion reporter gene. (C) Noninvasive imaging of transplanted mESCs with BLI and PET. Radiance is given in photons/sec per cm2/steradian. (Adapted from Cao et al. 2006 and reprinted with permission from Wolters Kluwer Health © 2006.)
Radiolabeled luciferase substrates have been explored for multimodality imaging of gene expression. In one report, a few 11C-labeled D-luciferin analogs were synthesized (Wang et al. 2006). PET studies showed a low retention of the 11C label at 45 min post-injection in luciferase-expressing tumors, whereas BLI with the unlabeled substrate D-luciferin and the radiolabeled analogs gave tumor signal within a few minutes of photon counting. Because D-luciferin and the radiolabeled analogs are substrates of Fluc, with a relatively high turnover rate, this is likely responsible for the poor retention of the tracers in the tumor. In addition, the required injected doses for these two imaging modalities also differ by several orders of magnitude. Therefore, although this is an interesting venue for exploring multimodality imaging, implementing it for optimal performance in animal studies is very challenging.
CONCLUSION
Recent advances in molecular imaging technologies have provided the potential for identifying changes at the genetic or molecular level long before they are detectable by conventional diagnostic techniques such as computed tomography or MRI. More importantly, these noninvasive imaging techniques can allow us to interrogate certain biological events (e.g., gene expression) in intact animals that previously could only be determined from in vitro assays of biopsied tissues or body fluids. To date, the vast majority of gene expression imaging studies has been in the preclinical stage. Although there are many gene therapy clinical trials currently ongoing, few of them have incorporated noninvasive imaging of gene expression into the trial.
The progress made over the last decade in the development of noninvasive imaging technologies for monitoring gene expression should allow molecular imaging to play a major role in the field of gene therapy (Min and Gambhir 2004). These tools have been validated in gene therapy models for longitudinal and quantitative monitoring of the location(s), magnitude, and time variation of gene delivery and/or expression. This article on optical imaging of gene expression is primarily applicable to preclinical research, and the emphasis has been mainly on the early studies that pioneered the field. The studies published to date clearly indicate that noninvasive imaging can accelerate the validation of preclinical models, which can give important insights toward clinical monitoring of human gene therapy. For clinical imaging of gene expression, PET holds the greatest promise (Yaghoubi et al. 2009).
Noninvasive optical imaging of gene expression has revolutionized biomedical research. It is no surprise that the 2008 Nobel Prize in Chemistry was awarded to three scientists for “the discovery and the development of the GFP.” Although both fluorescent and bioluminescent optical imaging have certain inherent disadvantages, such as poor tissue penetration and limitations as quantitative tools, their low cost, convenience, and constantly evolving imaging instrumentation, as well as emerging fluorescent proteins (Shaner et al. 2004; Shu et al. 2009) and luciferases (Loening et al. 2006, 2007) with better optical, physical, and biological characteristics, make these optical imaging methods irreplaceable in basic and preclinical research far beyond the field of imaging gene expression.
ACKNOWLEDGMENTS
We acknowledge financial support from the University of Wisconsin (UW) School of Medicine and Public Health’s Medical Education and Research Committee through the Wisconsin Partnership Program, the UW Carbone Cancer Center, the National Center for Research Resources 1UL1RR025011, and a Susan G. Komen Postdoctoral Fellowship (to H.H.).
- © 2011 Cold Spring Harbor Laboratory Press