Photoacoustic Imaging
Adapted from Imaging: A Laboratory Manual (ed. Yuste). CSHL Press, Cold Spring Harbor, NY, USA, 2010.INTRODUCTION
Photoacoustic imaging, which is based on the photoacoustic effect, has developed extensively over the last decade. Possessing many attractive characteristics such as the use of nonionizing electromagnetic waves, good resolution and contrast, portable instrumention, and the ability to partially quantitate the signal, photoacoustic techniques have been applied to the imaging of cancer, wound healing, disorders in the brain, and gene expression, among others. As a promising structural, functional, and molecular imaging modality for a wide range of biomedical applications, photoacoustic imaging can be categorized into two types of systems: photoacoustic tomography (PAT), which is the focus of this article, and photoacoustic microscopy (PAM). We first briefly describe the endogenous (e.g., hemoglobin and melanin) and the exogenous (e.g., indocyanine green [ICG], various gold nanoparticles, single-walled carbon nanotubes [SWNTs], quantum dots [QDs], and fluorescent proteins) contrast agents for photoacoustic imaging. Next, we discuss in detail the applications of nontargeted photoacoustic imaging. Recently, molecular photoacoustic (MPA) imaging has gained significant interest, and a few proof-of-principle studies have been reported. We summarize the current state of the art of MPA imaging, including the imaging of gene expression and the combination of photoacoustic imaging with other imaging modalities. Last, we point out obstacles facing photoacoustic imaging. Although photoacoustic imaging will likely continue to be a highly vibrant research field for years to come, the key question of whether MPA imaging could provide significant advantages over nontargeted photoacoustic imaging remains to be answered in the future.
BACKGROUND
More than a century ago, Alexander G. Bell first observed the photoacoustic effect. He found that absorption of electromagnetic waveforms, such as radio frequency (rf) or optical waves can generate transient acoustic signals in media. Such absorption leads to local heating and thermoelastic expansion, which can produce megahertz ultrasonic waves in materials. Because different biological tissues have different absorption coefficients, by measuring the acoustic signals with ultrasonic transducers it is possible to rebuild the distribution of optical energy deposition and ultimately obtain images of the biological tissues.
Photoacoustic imaging, based on the photoacoustic effect, is a noninvasive imaging modality, which has come a long way over the last decade (Wang 2008). Optical and rf waves, instead of electromagnetic waves at other wavelengths, are used in photoacoustic imaging because of their desirable physical properties, such as deeper tissue penetration and better absorption by contrast agents. The combination of high ultrasonic resolution with good image contrast because of differential optical/rf absorption is quite advantageous for imaging purposes (Xu and Wang 2006). When compared with fluorescence imaging, in which the scattering in tissues limits the spatial resolution with increasing depth, photoacoustic imaging has higher spatial resolution and deeper imaging depth, because scattering of the ultrasonic signal in tissue is much weaker. When compared with ultrasound imaging, in which the contrast is limited because of the mechanical properties of biological tissues, photoacoustic imaging has better tissue contrast, which is related to the optical properties of different tissues. In addition, the absence of ionizing radiation also makes photoacoustic imaging safer than other imaging techniques, such as computed tomography and radionuclide-based imaging techniques.
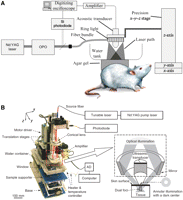
Typically, the spatial resolution provided by current photoacoustic-imaging systems is ∼100 µm, which may be further improved in the future (Debbage and Jaschke 2008). Given that optical absorption can reveal various physiological parameters, such as hemoglobin/melanin/water/ion concentration and oxygen saturation in living subjects, photoacoustic imaging is a promising structural, functional, and molecular imaging modality for a wide range of biomedical applications (Wang et al. 2003b; Li et al. 2008; Wang 2008). Photoacoustic-imaging systems can be categorized into two types: photoacoustic tomography (PAT; also referred to as optoacoustic tomography or thermoacoustic tomography) and photoacoustic microscopy (PAM) (Fig. 1). Although PAM has gained significant attention over the last several years (Maslov et al. 2005, 2008; Zhang et al. 2007; Hu et al. 2009; Stein et al. 2009a; Xie et al. 2009), we will primarily focus on PAT in this article. Both nontargeted and molecularly targeted photoacoustic imaging will be discussed.
Typical setups for photoacoustic tomography (A) and photoacoustic microscopy (B). (OPO) Optical parametric oscillator; (AD) analog to digital. (Reprinted from Zhang et al. 2006, with permission from Macmillan © 2006, and from De la Zerda et al. 2008, with permission from Macmillan © 2008.)
CONTRAST AGENTS FOR PHOTOACOUSTIC IMAGING
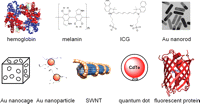
An ideal scenario for photoacoustic imaging would be that light absorption of normal tissue should be low for deeper signal penetration, whereas the absorption for the object of interest should be high for optimal image contrast (Xiang et al. 2009). The contrast agents used for photoacoustic imaging can be categorized into two types: endogenous and exogenous contrast agents. Certain endogenous molecules, such as hemoglobin and melanin (Fig. 2), have much stronger light absorption than normal tissue in both the visible and the near-infrared (700–900 nm) regions. Two of the biggest advantages of using endogenous contrast agents for imaging applications are safety and the possibility of revealing the true physiological condition, because the physiological parameters do not change during image acquisition if a relatively slow biological process is imaged.
A wide variety of contrast agents can be used for photoacoustic-imaging applications. (Au) Gold.
In many scenarios, such as the detection of early stage tumors, an endogenous contrast agent alone is insufficient to provide useful information. Because the intensity of a photoacoustic signal in biological tissue is proportional to optical energy absorption, which is proportional to the amount of the contrast agent (Rajian et al. 2009), exogenous contrast agents are frequently needed to provide better signal/contrast for photoacoustic imaging. Commonly used contrast agents for photoacoustic imaging include ICG, various gold nanoparticles (Yang et al. 2007), SWNTs (Hong et al. 2009; Pramanik et al. 2009a), QDs (Shashkov et al. 2008), and fluorescent proteins (Fig. 2).
NONTARGETED PHOTOACOUSTIC IMAGING
To date, photoacoustic imaging has generally been used in preclinical research and animal studies. Here, we will first give an overview of the studies using endogenous contrast agents (i.e., hemoglobin and melanin). Then, we will discuss the reports that used the various types of exogenous contrast agents mentioned above. Although none of these studies uses any targeting moiety, with the assistance of proper contrast agents, photoacoustic imaging could be used in many aspects of biomedical research.
Photoacoustic Imaging with Hemoglobin and/or Melanin
Hemoglobin and melanin are the two most important naturally occurring contrast agents for enhanced photoacoustic imaging. As an endogenous contrast agent, hemoglobin has been explored for photoacoustic imaging in a number of experimental scenarios such as visualizing brain structure and lesions (Wang et al. 2003a), delineating tumor vasculature (Siphanto et al. 2005), monitoring hemodynamics (Wang et al. 2006), imaging small animals (Kruger et al. 2003), and measuring microvascular blood flow (Fang et al. 2007).
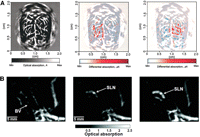
Photoacoustic imaging with hemoglobin can greatly facilitate brain-and-blood dynamics-related research (Wang et al. 2003a,b; Kolkman et al. 2004). In 2003, structures of the rat brain were accurately mapped with photoacoustic imaging (Fig. 3A; Wang et al. 2003b). Not only were the functional cerebral hemodynamic changes in cortical blood vessels around the whisker-barrel cortex evaluated in response to whisker stimulation, hyperoxia-induced and hypoxia-induced cerebral hemodynamic changes were also imaged. This pioneering study represented an important milestone for photoacoustic imaging, suggesting that this imaging modality holds promise for a number of applications in neurophysiology, neuropathology, and neurotherapy.
Nontargeted photoacoustic imaging. (A) Functional PAT imaging of cerebral hemodynamic changes in response to whisker stimulation. (Left) A PAT image of the vascular pattern in the superficial layer of the rat cortex acquired with the skin and the skull intact. (Middle) Noninvasive functional PAT images corresponding to left-side whisker stimulation. (Right) Noninvasive functional PAT images corresponding to right-side whisker stimulation. (B) Photoacoustic images acquired before (left), 5 min after (middle), and 140 min after (right) gold nanocage injection for sentinel lymph node (SLN) mapping in live animals. (BV) Blood vessel. (Adapted from Wang et al. 2003b with permission from Macmillan © 2003 and adapted from Song et al. 2009, with permission from American Chemical Society © 2009.)
Subsequently, the pattern of optical absorption in a mouse brain was also imaged (Wang et al. 2003a). The intrinsic tissue contrast revealed not only blood vessels, but also other brain structures such as the cerebellum, hippocampus, and ventriculi lateralis, which corresponded well with brain histology. Recently, noninvasive, high-resolution imaging of mouse brain activity has been reported (Stein et al. 2009b). With endogenous hemoglobin as the contrast agent, a contrast-to-noise ratio of 25 dB (decibels) was achieved. Such noninvasive visualization of the brain’s vascular system could be of great importance for studying the function of the brain, diagnosing possible disorders, and providing clinically translatable insights into the progression of various human neurological diseases.
Hemoglobin-based photoacoustic imaging can also facilitate the monitoring of many other biological processes such as burn recovery. For example, a two-wavelength photoacoustic-imaging technique was used to discriminate coagulated and noncoagulated blood in a dermal burn phantom (Talbert et al. 2007). Different optical absorption spectra of coagulated and noncoagulated blood produced different ratios of the peak photoacoustic amplitude, which could be analyzed to identify the different blood samples. Recently, multiwavelength photoacoustic measurement was performed to monitor the wound-healing process of extensive deep dermal burns in rats (Aizawa et al. 2008). The peak of the photoacoustic signal at 532 nm, an isosbestic point for oxyhemoglobin and deoxyhemoglobin, was found to shift to a shallower region of the injured skin tissue over time. Histological analysis showed that the photoacoustic signals reflected angiogenesis (the formation of new blood vessels) in the wound, indicating that multiwavelength photoacoustic measurement could be useful for monitoring the changes in local hemodynamics during wound healing.
Tumor growth is dependent on the formation of new blood vessels, and noninvasive imaging of tumor angiogenesis could play a pivotal role in many aspects of cancer patient management (Cai and Chen 2008; Cai et al. 2008b). Imaging of tumor angiogenesis is another important application for hemoglobin-based photoacoustic imaging (Ku et al. 2005). Photoacoustic imaging was used more than a decade ago to image blood vessels in highly scattering samples (Hoelen et al. 1998). It was shown that the sensitivity of this technique could reach single red blood-cell detection on a glass plate. In another study, photoacoustic images of tumor neovascularization were obtained over a 10-day period after subcutaneous inoculation of pancreatic tumor cells in rats (Siphanto et al. 2005). Three-dimensional data sets were acquired to visualize the development and to quantify the extent of individual blood vessels around the growing tumor, as well as to measure the blood concentration changes inside the tumor.
Photoacoustic imaging with melanin is mainly used for the diagnosis, prognosis, and treatment planning of melanotic melanoma (>90% of all melanomas) (Oh et al. 2006; Wang 2008). The combination of hemoglobin and melanin can be used for more accurate detection of melanomas, because more parameters can be measured. Dual-wavelength reflection-mode photoacoustic imaging has been used to noninvasively obtain three-dimensional images of subcutaneous melanomas and their surrounding vasculature in living nude mice (Oh et al. 2006). At the two wavelengths used for hemoglobin and melanin, 584 nm and 764 nm, respectively, the absorption coefficients of blood and melanin-pigmented melanomas vary greatly relative to each other. Therefore, in vivo photoacoustic imaging with a 764-nm light source was able to image the three-dimensional melanin distribution inside the skin to a maximum thickness of 0.5 mm of melanoma. In another study, photoacoustic imaging was reported to be capable of detecting melanoma cells within the human circulation system (Weight et al. 2006).
Photoacoustic imaging may also be potentially useful in visualizing certain breast cancers based on intrinsic optical absorption contrast, and proof-of-principle studies have been reported (Manohar et al. 2007; Pramanik et al. 2008). However, despite all of the progress made to date by photoacoustic imaging with hemoglobin and/or melanin, the use of exogenous contrast agents would undoubtedly benefit this imaging modality by enhancing the image quality and contrast, particularly in cancer research. To date, the majority of contrast agents for photoacoustic imaging are not molecularly targeted.
ICG-Based Photoacoustic Imaging
ICG, a tricarbocyanine dye with peak absorption at ∼800 nm, has been used as a diagnostic aid for measuring blood volume, cardiac output, or hepatic function. Noninvasive photoacoustic angiography of animal brains has been reported with ICG (Wang et al. 2004). When it is injected into the circulation system of a rat, ICG significantly enhances the absorption contrast between the blood vessels and the background tissues. Because near-infrared light can penetrate deep into brain tissues through the skin and the skull, the vascular distribution in the rat brain was successfully reconstructed from the photoacoustic signals with high spatial resolution and low background. Subsequently, it was reported that PAT could image objects embedded at depths of up to 5 cm at a resolution of <1 mm with a sensitivity of <10 pm of ICG in the blood (Ku and Wang 2005). The resolution was found to deteriorate slowly with increasing imaging depth.
Gold Nanoparticle-Based Photoacoustic Imaging
Gold nanoparticles (e.g., nanorods and nanocages) have been used for photoacoustic imaging (Cai and Chen 2007; Cai et al. 2008a). Initially, gold nanorods were proposed for photoacoustic flow measurements by the use of laser-induced shape transitions (Li et al. 2005). A series of studies were performed to investigate the shape dependence of the optical absorption of gold nanorods, as well as the shape transition induced by pulsed laser irradiation (Wei et al. 2006, 2007). It was found that photon-induced shape transition of gold nanorods involves mainly a rod-to-sphere conversion and a shift in the peak optical absorption wavelength. The application of laser pulses will induce shape changes in gold nanorods as they flow through a region of interest, and the quantitative flow information can be derived based on the photoacoustic signals (measured as a function of time) from the irradiated gold nanorods.
The plasmon resonance absorption and scattering of gold nanorods in the near-infrared region makes them attractive for in vivo imaging applications (Tong et al. 2009). In one study it was reported that 25 µL of gold nanorod solution with a concentration of 1.25 pm, when injected into nude mice, could be detected by a single-channel acoustic transducer (Eghtedari et al. 2007).
Current sentinel lymph-node (SLN) mapping methods, based on blue dye and/or nanometer-sized radioactive colloid injections, are intraoperative because of the need for visual detection of the blue dye and low spatial resolution of Geiger counters in detecting radioactive colloids. Compared with these techniques, photoacoustic mapping of SLNs with gold nanocages could have certain attractive features: noninvasiveness, strong optical absorption in the near-infrared region for deeper tissue penetration, and high-concentration accumulation of the gold nanocages. Recently, it was shown that gold nanocages could be used for noninvasive photoacoustic imaging of SLNs (Fig. 3B; Song et al. 2009). In an animal model, gold nanocage-containing SLNs, as deep as 33 mm below the skin surface, could be detected with good contrast. Potentially, these gold nanocages could also be conjugated with certain targeting moieties (e.g., antibodies or peptides) for molecular-imaging applications.
Photoacoustic Imaging with Other Nanoparticles
In addition to gold nanoparticles, two other types of nanoparticles could also be promising contrast agents for in vivo photoacoustic imaging: SWNTs and QDs. When compared with blood in phantom studies, SWNTs were found to show significant signal enhancement for PAT at a 1064-nm wavelength (Pramanik et al. 2009b). Recently, noninvasive SWNT-enhanced photoacoustic identification of SLNs in a rat model was reported (Pramanik et al. 2009a). The SLNs were successfully imaged in vivo with high contrast-to-noise ratio (>80) and good resolution (∼500 µm). Because SWNTs have optical absorption over a wide excitation wavelength range, the imaging depth could be maximized by varying the incident light wavelength to the near-infrared region, where biological tissues (e.g., hemoglobin, tissue pigments, lipids, and water) have low light absorption.
Over the last decade, QDs have become one of the fastest growing areas of research in nanotechnology (Cai et al. 2007b; Li et al. 2007b). The vast majority of QD-based research uses their fluorescent properties. Interestingly, the use of QDs for photoacoustic imaging has been described with a nanosecond pulse laser excitation (Shashkov et al. 2008). The laser-induced photoacoustic phenomena were studied with an advanced multifunctional microscope. It was shown that QDs could be used as photoacoustic contrast agents and sensitizers, thereby providing an opportunity for multimodal (photoacoustic and fluorescence) imaging and potentially also photothermal therapy.
MOLECULAR PHOTOACOUSTIC IMAGING
The field of molecular imaging—the visualization, characterization, and measurement of biological processes at the molecular and cellular levels in humans and other living systems (Mankoff 2007)—has expanded tremendously over the last decade. In general, molecular imaging modalities include molecular magnetic resonance imaging (MRI), magnetic resonance spectroscopy, optical bioluminescence, optical fluorescence, targeted ultrasound, single-photon emission computed tomography, and positron emission tomography (Massoud and Gambhir 2003). Continued development and wider availability of scanners dedicated to small animal imaging studies, which can provide a similar in vivo imaging capability in mice, primates, and humans, can enable the smooth transfer of knowledge and molecular measurements between species, thereby facilitating clinical translation.
Molecular imaging takes advantage of the traditional diagnostic imaging techniques and introduces molecular-imaging probes to measure the expression of indicative molecular markers at different stages of diseases. Noninvasive detection of various molecular markers of diseases can allow for much earlier diagnosis, earlier treatment, and better prognosis that will eventually lead to personalized medicine (Cai et al. 2006a, 2008b,c). Recently, MPA imaging has gained significant interest, and a few proof-of-principle studies have been reported. By incorporating a targeting moiety into the above-mentioned contrast agents, photoacoustic imaging can be used to obtain the molecular signatures of various diseases, in particular, cancer.
ICG-Based MPA Imaging
ICG-embedded nanoparticles (∼100 nm in diameter), using modified silicate as a matrix, have been developed as contrast agents for photoacoustic imaging (Kim et al. 2007). These ICG-embedded nanoparticles showed improved stability in aqueous solution when compared with the free dye. When conjugated with anti-HER-2 antibody, the nanoparticles showed high contrast and high efficiency for binding to prostate cancer cells in vitro. No in vivo MPA imaging with these ICG-embedded nanoparticles has been reported yet. The fact that ICG can also be used as a photosensitizer for photodynamic therapy may expand the future use of this agent, which can potentially combine both diagnostic and therapeutic functions in a single entity.
Gold Nanoparticle-Based MPA Imaging
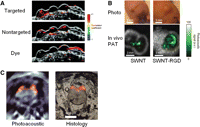
Targeted gold nanoparticles have been used mainly for molecular imaging and therapy of cancer (Cai et al. 2008a). Recently, it was reported that antibody-conjugated, epidermal growth-factor receptor (EGFR)-targeted spherical gold nanoparticles undergo molecular specific aggregation when they bind to EGFR on the cell surface, thereby leading to a redshift in their plasmon resonance frequency (Fig. 4A; Mallidi et al. 2009). Capitalizing on this effect, the efficacy of MPA imaging was shown using subcutaneous tumor-mimicking gelatin implants in excised mouse tissue, suggesting that selective and sensitive detection of cancer cells is potentially possible with multiwavelength photoacoustic imaging and molecular-specific gold nanoparticles.
Molecular photoacoustic imaging. (A) Correlation coefficient images, obtained by comparing multiwavelength photoacoustic images with optical spectra of targeted gold nanoparticles, nontargeted gold nanoparticles, and a dye, overlaid on ultrasound images of the subcutaneous gelatin implants in mouse tissue ex vivo. Only correlation coefficient values >0.75 were displayed in the images. Images measure 44 mm laterally and 9.1 mm axially. (B) Photographs of the tumors in mice and the corresponding photoacoustic subtraction images (green) shown as horizontal slices through the tumors. (C) (Left) Photoacoustic image of the zebrafish brain with fluorescent protein expression shown in color. (Right) Corresponding histology of a dissected fish. See text for details. (Adapted from De la Zerda et al. 2008, with permission from Macmillan © 2008, adapted from Mallidi et al. 2009, with permission from American Chemical Society © 2009, and adapted from Razansky et al. 2009, with permission from Macmillan © 2009.)
The potential of gold nanoparticle-based MPA imaging in the monitoring of antitumor necrosis factor (anti-TNF) therapy has been evaluated (Chamberland et al. 2008). In this study, the contrast agent is composed of gold nanorods conjugated with Etanercept molecules, a fusion protein of the soluble TNF receptor and the Fc component of human immunoglobulin G1 (Cao et al. 2007). In ex vivo studies, it was found that gold nanorods with a concentration down to 1 pm in phantoms or 10 pm in biological tissues could be imaged with good signal-to-noise ratio and high spatial resolution (Chamberland et al. 2008). Further investigations are warranted to test this agent for in vivo MPA imaging.
One of the attractive features of a gold nanorod is its tunable optical absorption property, which is dependent on its size and its aspect ratio (i.e., the length divided by the width). The use of gold nanorods for MPA imaging with simultaneous multiple targeting has been reported (Li et al. 2008). Two types of nanorods with different peak absorption wavelengths were each conjugated with anti-EGFR or anti-HER-2 antibody, respectively. By simply switching the wavelength of the excitation laser, multiple molecular signatures could be obtained both in vitro and in vivo. Future studies with more gold nanorods/nanoparticles with different optical properties may potentially allow for simultaneous visualization of even more cancer-related molecular targets.
SWNT-Based MPA Imaging
The SWNT is perhaps the most successful contrast agent that has been used for MPA imaging. Integrin αvβ3 and arginine-glycine-aspartic acid (RGD), a potent integrin αvβ3 antagonist (Cai and Chen 2006), is one of the most extensively studied and validated receptor-ligand pairs (Cai and Chen 2008; Cai et al. 2008b). In a recent study, SWNT conjugated with cyclic RGD peptides was used as a contrast agent for MPA imaging of tumors in living mice (Fig. 4B; De la Zerda et al. 2008). Intravenous administration of the SWNT-RGD conjugate to tumor-bearing mice showed an eight times greater photoacoustic signal in the tumor than the photoacoustic signal shown in tumor-bearing mice injected with nontargeted SWNTs. Taking advantage of the intrinsic Raman signal of the SWNT, the in vivo MPA imaging results were further validated ex vivo with Raman microscopy. With regard to sensitivity, a concentration of 50 nm of SWNTs was found to produce a photoacoustic signal equivalent to the mouse tissue (the background signal) in this study (De la Zerda et al. 2008). However, the minimum detectable concentration of SWNTs is likely less than that because the photoacoustic images were acquired before and after the administration of the contrast agent, which makes it possible to separate the signal of the SWNT from the background.
Targeted SWNTs can be used for both MPA imaging and photoacoustic therapy. Recently, the large photoacoustic effect of SWNTs was explored for targeting and selective destruction of cancer cells (Bin et al. 2009). Under the irradiation of a 1064-nm Q-switched millisecond pulsed laser, SWNTs showed a large photoacoustic effect in suspension, which could trigger an explosion at the nanoscale. By conjugating the SWNTs with folic acid, which can bind to cancer cells overexpressing the folate receptor on the membrane, the laser power used for cancer cell killing could be reduced 150–1500 times. This discovery has opened up new perspectives for exploring the photoacoustic properties of SWNTs in cancer therapy.
MPA Imaging of Gene Expression
In one interesting study, the first demonstration of PAT for reporter gene imaging was reported (Li et al. 2007a). Rats inoculated with 9L/lacZ gliosarcoma tumor cells were imaged with PAT before and after injection of X-gal, an optically transparent lactose-like substrate, which yields a stable dark-blue product following the cleavage of the glycosidic linkage by β-galactosidase (the protein encoded by lacZ). A spatial resolution of ∼400 µm, as well as a sensitivity of ∼0.5 µm, were achieved. With the future development of new absorption-based reporter gene systems, MPA imaging is expected to become a valuable technique in molecular imaging research.
Fluorescent proteins are widely used in cell biology and in the study of transgenic animals, because they can be genetically targeted to a specific molecule of interest (Giepmans et al. 2006). One major advantage of using fluorescent proteins as the contrast agent for photoacoustic imaging is that they overcome the limitations of conventional fluorescence microscopy and allow imaging of gene expression in much deeper tissues (Burgholzer et al. 2009).
Recently, it was reported that multispectral PAT can achieve tissue penetration of several millimeters (potentially centimeters) with a resolution of 20–100 µm, which remains constant as a function of depth and depends only on the ultrasonic detector characteristics (Razansky et al. 2009). This technique is capable of visualizing fluorescent proteins in small living organisms (Fig. 4C). Whole-body imaging of Drosophila melanogaster pupae and adult zebrafish revealed that tissue-specific expression of different fluorescent proteins could be resolved for precise morphological and functional observations in vivo. This report represents an important step forward in high-resolution imaging of fluorescent proteins expressed in genetically manipulated organisms.
Multimodality Molecular Imaging
Among all of the molecular imaging modalities, no single modality is perfect and sufficient to obtain all of the necessary information for a given question. For example, it is difficult to accurately quantify fluorescence signals in living subjects with fluorescence imaging alone, particularly in deep tissues; MRI has exquisite soft-tissue contrast, yet it suffers from very low sensitivity. Combining imaging modalities can offer synergistic advantages more than any modality alone. In several reports, photoacoustic imaging has been used together with other imaging modalities to obtain complementary information.
Because of the overwhelming scattering of light in biological tissues, the spatial resolution and imaging depth of conventional fluorescence imaging is unsatisfactory. Dual-modality imaging, with both fluorescence and PAT, was found to be beneficial in an animal tumor model (Wang et al. 2005). An integrin αvβ3-targeted peptide, conjugated to ICG, was used as the molecular probe for tumor detection in nude mice inoculated with M21 human melanoma cell lines (integrin αvβ3-positive). PAT was able to provide noninvasive images of both the brain structure and the angiogenesis associated with tumor growth, whereas fluorescence imaging offered high sensitivity for tumor detection. Further, coregistration of the PAT and fluorescence images enabled simultaneous visualization of the tumor location, angiogenesis, and brain structure.
Another recent report explored the combination of PAT with MRI (Bouchard et al. 2009). The probe used in this study consists of ferromagnetic cobalt particles coated with gold, which provides both biocompatibility and a unique shape that enables optical absorption over a broad range of frequencies. This dual-modality agent was shown to be useful for detecting trace amounts of the nanoparticles in biological tissues, in which MRI provides volume detection and PAT performs edge detection. Modification of this dual-modality imaging agent with a targeting ligand should be pursued in future studies.
CONCLUSION
Photoacoustic imaging possesses many attractive characteristics: the use of nonionizing electromagnetic waves, good resolution and contrast, portable instrumentation, and the capability of quantitating the signal to a certain extent. Big strides have been made over the last decade, and many diseases can be investigated with photoacoustic imaging, such as cancer, wound healing, and disorders in the brain, among others. PAT compares very favorably to other imaging modalities with its precise depth information, submillimeter resolution, and nanomolar sensitivity. With further improvement in background reduction and hardware/software, as well as the use of lasers with high-repetition rates, it is likely that PAT will find wide uses in the future in both basic research and clinical care. While the photoacoustic research community continues to discover new phenomena and to invent new technologies, several companies are actively commercializing the instrument for PAT. Therefore, PAT will continue to be a highly vibrant research field in the years to come.
A significant portion of the contrast agents used for photoacoustic imaging is based on certain nanoparticles. Although nanoparticles offer many advantages over conventional small molecule-based imaging/therapeutic agents, such as multifunctionality and flexibility, many barriers exist for in vivo applications in preclinical animal models and future clinical translation of these nanoparticles. These include biocompatibility, in vivo kinetics, (tumor) targeting efficacy, acute/chronic toxicity, the ability to escape the reticuloendothelial system, and cost effectiveness.
In terms of molecularly targeted photoacoustic imaging, (tumor) vasculature targeting is the best bet for nanoparticle-based contrast agents, because many of these nanoparticles are too large to extravasate (Cai et al. 2006b, 2007a). A circulation half-life of several hours may be sufficient to allow for efficient (tumor) vasculature targeting. Close partnerships among scientists in various disciplines (e.g., engineering, chemistry, oncology, physics, and biology, just to name a few) are needed to move the field forward in a timely manner. Although several proof-of-principle studies have been reported, whether MPA imaging can provide significant advantages over nontargeted photoacoustic imaging remains to be shown.
ACKNOWLEDGMENTS
We acknowledge financial support from the University of Wisconsin (UW) School of Medicine and Public Health’s Medical Education and Research Committee through the Wisconsin Partnership Program, the UW Carbone Cancer Center, NCRR 1UL1RR025011, and a Susan G. Komen Postdoctoral Fellowship (to H.H.).
- © 2011 Cold Spring Harbor Laboratory Press