Combining Optogenetics and Electrophysiology to Analyze Projection Neuron Circuits
- 1Department of Physiology, Feinberg School of Medicine, Northwestern University, Chicago, Illinois 60611;
- 2McGovern Institute for Brain Research, Massachusetts Institute of Technology, Cambridge, Massachusetts 02139
Abstract
A set of methods is described for channelrhodopsin-2 (ChR2)-based synaptic circuit analysis that combines photostimulation of virally transfected presynaptic neurons’ axons with whole-cell electrophysiological recordings from retrogradely labeled postsynaptic neurons. The approach exploits the preserved photoexcitability of ChR2-expressing axons in brain slices and can be used to assess either local or long-range functional connections. Stereotaxic injections are used both to express ChR2 selectively in presynaptic axons of interest (using rabies virus [RV] or adeno-associated virus [AAV]) and to label two types of postsynaptic projection neurons of interest with fluorescent retrograde tracers. In brain slices, tracer-labeled postsynaptic neurons are targeted for whole-cell electrophysiological recordings, and synaptic connections are assessed by sampling voltage or current responses to light-emitting diode (LED) photostimulation of ChR2-expressing axons. The data are analyzed to estimate the relative amplitude of synaptic input and other connectivity parameters. Pharmacological and electrophysiological manipulations extend the versatility of the basic approach, allowing the dissection of monosynaptic versus disynaptic responses, excitatory versus inhibitory responses, and more. The method enables rapid, quantitative characterization of synaptic connectivity between defined pre- and postsynaptic classes of neurons.
MATERIALS
Reagents
3-((R)-2-carboxypiperazin-4-yl)-propyl-1-phosphonic acid (CPP) or other N-methyl-d-aspartate (NMDA) antagonist (optional; see Step 4)
Artificial cerebrospinal fluid (ACSF) for LSPS
-
ACSF is used as the bath solution. For analysis of monosynaptic inputs (see Step 9 and Table 1), add tetrodotoxin (TTX, 1 µm) and 4-aminopyridine (4AP, 100 µm).
Recording conditions for isolating different kinds of signals
Internal (pipette) solution containing desired cation (see Table 1)
Mice of desired genotype and developmental stage
Retrograde tracers (see Step 2)
-
Examples include fluorescent microspheres (red or green RetroBeads [Lumafluor]) and cholera toxin subunit B conjugated with fluorophores (Molecular Probes).
Viruses encoding ChR2 (see Step 1)
-
Commercial sources include Penn Vector Core (for AAV-ChR2) and Duke Viral Vector Core (for RV-ChR2); protocols are also available for high-titer preparation of RV (see Protocol: Rabies Viral Vectors for Monosynaptic Tracing and Targeted Transgene Expression in Neurons [Wickersham and Sullivan 2015])
Equipment
Pipette puller and microgrinder
Slice rig equipped for whole-cell recording, photostimulation with a blue LED and/or laser, and epifluorescence microscopy
Software packages (e.g., Ephus [http://scanimage.vidriotechnologies.com]) for hardware control and data acquisition
Stereotaxic frame and micromanipulator
METHOD
-
See Figure 1 for an outline of the experimental procedures.
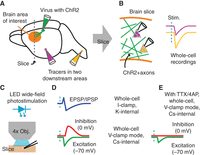
General paradigm for optogenetic circuit electroanatomy. (A) In vivo injections are made to deliver either rabies or adeno-associated virus carrying ChR2 to one brain region of interest that is either upstream or downstream from the main region of interest (orange area indicates where brain slices will be made), and to deliver retrograde tracers of two different colors in areas that are downstream from this area. (B) In brain slices of the main brain area of interest, ChR2-expressing presynaptic axons (green) are photostimulated while recordings are made from the two types of projection neurons identified by fluorescent labeling. (C) LED illumination through a low-power objective (Obj.) is used for wide-field photostimulation. (D) Electrophysiological manipulations allow different types of responses to be sampled, including mixed excitatory and inhibitory postsynaptic potentials (EPSP/IPSP) in current-clamp (I-clamp) mode, or isolated inhibitory and excitatory currents in voltage-clamp (V-clamp) mode. “K-internal” and “Cs-internal” refer to the cation used in the internal (pipette) solution; see Table 1. (E) Pharmacological manipulations allow isolation of purely monosynaptic inputs.
In Vivo Labeling of Pre- and Postsynaptic Neurons
-
Perform in vivo stereotaxic delivery of virus (Step 1) and retrograde tracers (Step 2) into mice following standard procedures (Cetin et al. 2006). For reviews and further descriptions of viral and nonviral tracing methods, see Wickersham et al. (2007), Betley and Sternson (2011), Nassi et al. (2015), and Protocol: Rabies Viral Vectors for Monosynaptic Tracing and Targeted Transgene Expression in Neurons (Wickersham and Sullivan 2015).
-
1. Inject ChR2-virus following one of the two paradigms described below, depending on the goals of the experiment.
-
With either paradigm, the infected neurons’ ChR2-expressing axons will be the presynaptic source of input in the subsequent in vitro experiments, as they remain photoexcitable in brain slices (Petreanu et al. 2007). Viral constructs should be chosen to allow coexpression of a fluorescent protein (e.g., Venus, mCherry, etc.) to aid in targeting subsequent recordings to regions containing transfected axons. Choice of virus strain or serotype is also an important consideration, influencing factors such as cytotoxicity in the case of RV, the degree of retrograde labeling in the case of AAV, and more (Nassi et al. 2015; Reardon et al. 2016).
-
i. Use injection with RV-ChR2 to optogenetically label one or the other of two classes of projection neurons that are intermingled in the same brain region, as a way to study their local interconnections (Kiritani et al. 2012).
-
The projection neurons are retrogradely infected by injecting rabies viruses (RV) encoding ChR2 (Osakada et al. 2011; Kiritani et al. 2012) into their axonal projections (i.e., downstream brain area).
-
Alternatively, mouse lines are available that express Cre recombinase in various types of projection neurons (Gerfen et al. 2013). In this case, inject AAV carrying Cre-dependent ChR2 into the cortical area of interest to label a small cluster of neurons. A consideration is the potential damage from craniotomy and insertion of a pipette in the same brain region where recordings will subsequently be performed. Grinding the injection pipettes to a sharp beveled edge on a microgrinder and inserting pipettes at an angle to the cortical surface helps to minimize damage.
-
-
ii. Use injection with AAV-ChR2 to study long-range afferent innervation to two types of projection neurons that are intermingled in the same brain region.
-
The long-range axons of projection neurons in an upstream area of interest can be anterogradely labeled by infecting their parent somata with AAV virus encoding ChR2 (e.g., Petreanu et al. 2009; Hooks et al. 2013).
-
-
-
2. Inject two different retrograde tracers.
-
Stereotaxically inject these as appropriate to label two classes of projection neurons of interest. The somata of the labeled neurons will be targeted as the postsynaptic neurons in the subsequent in vitro experiment. The temporal order of the tracer and viral injections is generally not critical.
-
Alternatively, injection of only one retrograde tracer suffices for experiments where synaptic inputs will be compared between identified projection neurons at different spatial locations; for example, vertically across layers in a particular cortical area, horizontally along a layer across the cortex, across subcortical nuclei (including over multiple slices), etc. (Suter and Shepherd 2015; Yamawaki and Shepherd 2015).
-
Analysis of Excitatory and Inhibitory Currents
-
Analysis of excitatory and inhibitory currents is described in Steps 3–8. For analysis of monosynaptic circuits, see Step 9.
In Vitro Circuit Analysis of Disynaptic Currents
-
3. After a suitable time for transgene expression, typically ∼1 wk for RV-ChR2 and ∼3 wk for AAV-ChR2 (Kiritani et al. 2012; Yamawaki and Shepherd 2015), follow standard methods for preparing brain slices for photostimulation experiments (see Protocol: Circuit Mapping by Ultraviolet Uncaging of Glutamate [Shepherd 2012]).
-
The wait time is shorter with RV both because transgene expression levels increase rapidly after infection and because cytotoxicity eventually develops and kills infected cells. Control experiments should be performed to assess the health (e.g., intrinsic electrophysiological properties) of RV-infected neurons in the time window of recordings (Kiritani et al. 2012). Other retrograde viruses, such as rabies-pseudotyped lentivirus, may be useful alternatives if longer survival times are needed (see Protocol: Lentiviral Vectors for Retrograde Delivery of Recombinases and Transactivators [Wickersham et al. 2015]).
-
-
4. Place a brain slice in the recording chamber perfused with artificial cerebrospinal fluid (ACSF, warmed to 32°C with an in-line heater).
-
Pharmacological conditions and other experimental parameters can be set to isolate different kinds of signals of interest for circuit analysis (Table 1). NMDA-receptor blockade (e.g., CPP, 1 µm) is commonly used to prevent synaptic plasticity.
-
-
5. Under a high-power objective lens, target postsynaptic neurons based on fluorescent retrograde labeling, and establish a gigaohm seal using a pipette filled with cesium- or potassium-based internal solution (according to the goal of the experiment; see Table 1).
-
6. Perform recording from the first neuron as follows:
-
i. Switch to a low-power objective lens (e.g., 4×, so that photostimuli will broadly excite axons across the neuropil around the neuron’s dendrites). Adjust the position of the slice to bring it into an appropriate alignment within the field of view, noting the x–y coordinates so that they can be used for subsequent neurons in the same slice.
-
ii. Compensate pipette capacitance and rupture the membrane for whole-cell configuration in voltage-clamp (V-clamp) or current-clamp (I-clamp) mode, depending on the goals of the experiment.
-
iii. For routine mapping of excitatory inputs, photostimulate the presynaptic axons while recording from the postsynaptic neuron by driving an LED with a brief (e.g., 5-msec) pulse to deliver a wide-field flash of blue light.
-
For the first neuron of the slice, choose one that is expected to receive relatively strong input (based on previous experiments). Start with high intensity (e.g., 1 mW/mm2), and adjust as needed to obtain (if possible) a response of moderate amplitude (e.g., 100 pA). Then use this LED power setting as the standard level for all following neurons in the same slice. Sampling at additional LED powers can also be useful, particularly in the early stages of a project, to assess how responses scale with stimulus intensity. A common practice is to acquire several traces for online or posthoc averaging, with interstimulus intervals set to allow recovery from desensitization.
-
-
iv. To isolate excitatory currents, apply a command voltage to hold near the GABAergic reversal potential, typically around −70 mV as calculated or determined empirically, such as by GABA uncaging (Wood et al. 2009).
-
Inhibitory currents can similarly be sampled at the glutamatergic reversal potential of ∼0 mV (usually with cesium-based internal solution, for better voltage control). For cortical slices, GABA antagonists should be avoided as photostimulation of excitatory neurons may generate epileptiform discharges, even at low doses (Weiler et al. 2008).
-
-
-
7. Record from a second, neighboring neuron labeled with the other retrograde tracer, and repeat the photostimulation recording. Use the same x–y coordinates for the slice position, and the same LED power setting(s), so that the same axons are stimulated as for the first neuron. Repeat for additional neurons from the same slice, as needed for the objectives of the experiment.
-
8. Continue to Step 10 (statistical analysis).
In Vitro Circuit Analysis of Monosynaptic Inputs
-
9. Perform circuit analysis as in Steps 3–8, except include tetrodotoxin (TTX, 1 µm) and 4-aminopyridine (4AP, 100 µm) in the bath solution.
-
Bath solution containing TTX and 4AP abolishes action potentials, enhances local depolarization of photostimulated ChR2-expressing axons, and results in local photoevoked depolarization of presynaptic terminals sufficient to induce neurotransmitter release—isolating purely monosynaptic inputs (Petreanu et al. 2009). The TTX/4AP conditions were originally developed for use with laser scanning photostimulation (subcellular ChR2-assisted circuit mapping, or “sCRACM”) (Petreanu et al. 2009), but can be used with LED photostimulation to map cellular connections to retrogradely labeled projection neurons (Suter and Shepherd 2015; Yamawaki and Shepherd 2015). The LED and laser optical systems can be implemented in the same microscope.
-
Statistical Analysis
-
10. Analyze the traces offline to quantify the responses.
-
The relative innervation of two postsynaptic cell types by a single presynaptic axon type can be shown by plotting the response amplitudes (e.g., mean or peak) against each other. Pairwise and other types of comparisons can be made using a variety of statistical measures to analyze inputs to the two types of postsynaptic projection neurons (Yamawaki and Shepherd 2015). For example, for paired observations, use the nonparametric sign test for two medians (Kanji 2006).
-
In general, the normalization-based approach provides a measure of the relative amplitude of synaptic input to multiple postsynaptic neurons. It controls for variability in factors that can affect the absolute amplitude, such as the number of infected neurons per animal and expression levels of ChR2 in axons. The method can be used to compare neurons not only in the same slice but also in different slices from the same animal (Yamawaki and Shepherd 2015). Normalized responses can then be pooled across experiments (i.e., animals) for group (population) analyses.
-
DISCUSSION
Projection neurons, through their interconnections, form the major circuits of the central nervous system. Brain regions typically contain multiple classes of projection neurons, and thus two basic questions about projection neuron circuits are the extents to which different projection classes (1) interconnect locally and (2) receive inputs from upstream regions (“long-range inputs”). The preserved photoexcitability of ChR2-expressing axons in brain slices has ushered in a new era of optogenetic-electrophysiological experiments capable of probing local and long-range connectivity (Petreanu et al. 2007). The presynaptic photostimulation aspect of the protocol described here builds on methods originally described by Petreanu, Svoboda, and colleagues (Petreanu et al. 2007, 2009). A simplification is that connectivity is assessed with an LED light source, which is technically simpler to implement than a laser scanning system and also substantially shortens the recording time per cell, allowing more recordings per slice. An LED and a laser scanning system can both be mounted on the same microscope, enabling the operator to use both methods in the same experiment, including for the same cell. On the postsynaptic side, the key aspect of this protocol is the use of retrograde tracers such as fluorescent microspheres (Katz et al. 1984) to allow identified projection neurons to be targeted for postsynaptic recordings during photostimulation of presynaptic ChR2-expressing axons (Anderson et al. 2010; Mao et al. 2011).
The general paradigm of making pairwise comparisons of electrophysiological responses recorded in two different postsynaptic cell types following photostimulation of presynaptic inputs has been used in a wide variety of experiments. Here, by recording from identified projection neurons, the circuit analysis is extended to include the areas downstream from where the recordings are performed—providing an additional and functionally relevant level of detail in circuit diagrams. For example, detection of excitatory inputs from primary motor cortex (M1) to M1-projecting neurons in secondary somatosensory cortex indicates a pattern of recurrent connectivity between the two areas (Suter and Shepherd 2015).
The basic approach described here should be easy to adapt for many different types of experiments. Using a laser scanning system (and adding TTX and 4AP in the bath solution) allows high-resolution mapping of the dendritic location of presynaptic inputs, as discussed above (Petreanu et al. 2009). Alternatively, with a laser scanning system but with TTX/4AP omitted from the bath, one can generate high-frequency barrages of inputs (from axons at different locations) to study how identified postsynaptic projection neurons translate synaptic input into spiking output; for example, this approach showed a role for hyperpolarization-activated, cyclic nucleotide–gated (HCN) channels in integrating synaptic input from layer 2/3 pyramidal neurons to generate action potentials in corticospinal neurons in motor cortex (Sheets et al. 2011).
While the optogenetic-electrophysiological approach has key advantages due to the ability to selectively activate just the presynaptic axons of interest, it does have several limitations. For one, the approach described here enables one to determine the relative strength of input to different neurons from the same source of input, but unlike paired recordings does not provide a measure of absolute input strength—not only because multiple axons are photoexcited, but because ChR2-related factors (e.g., slow kinetics, Ca2+ permeability) can affect release from presynaptic terminals (Schoenenberger et al. 2011). The use of high-frequency repetitive photostimulation (at the same location) is potentially an issue, due to ChR2 desensitization and the slow kinetics of ChR2 (compared to those of potassium channels involved in repolarization), which can distort presynaptic waveforms and affect short-term plasticity (Zhang and Oertner 2007). However, these problems have been reduced in newer variants of ChR2 with faster kinetics (Gunaydin et al. 2010; Berndt et al. 2011; Kleinlogel et al. 2011; Klapoetke et al. 2014), and in some cases may be avoided by photostimulating axons at a short distance away from the recorded postsynaptic neuron (Little and Carter 2013; Jackman et al. 2014).
Footnotes
-
↵3 Correspondence: g-shepherd{at}northwestern.edu
-
From the Ion Channels collection, edited by Paul J. Kammermeier, Ian Duguid, and Stephan Brenowitz.
- © 2016 Cold Spring Harbor Laboratory Press