Assessing the Immune Response When Raising Antibodies for Use in Xenopus
- European Xenopus Resource Centre, Institute of Biological and Biomedical Sciences, University of Portsmouth, Portsmouth, Hampshire PO1 2DY, United Kingdom
- ↵1Correspondence: matthew.guille{at}port.ac.uk
Abstract
Frog-specific antibodies usually must be raised for work in Xenopus. Selecting a host animal whose immune system will respond to a target antigen with an antibody response is essential to obtaining high-quality antibodies. To determine whether an immunized animal has produced antibodies against an antigen, western blotting using Xenopus embryo or egg extract as the protein source can be performed as described here. When a protein of the expected size is detected by western blotting in the immune sera but not the preimmune sera, the antibody has been successfully raised.
MATERIALS
Reagents
1,1,1-trichlorotrifluorethane (optional; see Step 4)
Antibodies (select one of the following; see Steps 13–14)
-
Primary antibody (HRP-conjugated)
-
Primary antibody (unconjugated) and species-appropriate HRP-conjugated secondary antibody
-
See Protocol: Raising Antibodies for Use in Xenopus (Piccinni and Guille 2020a). To reduce background on the western blot, the antibodies can be purified as described in Protocol: Purifying Antibodies Raised against Xenopus Peptides (Piccinni and Guille 2020b).
Blocking buffer (select one of the following based on empirical testing)
-
1% Blocking Reagent (Roche 11096176001) in maleic acid buffer (100 mm maleic acid, 150 mm NaCl)
-
5% BSA (Fisher Scientific BPE9701) in TBSTw
-
5% (w/v) nonfat dry milk (Marvel) in TBSTw
-
Some blocking buffers work better than others for certain antibodies.
dH2O
Detection buffers
-
ECLI buffer for chemiluminescent detection of HRP-conjugated antibodies
-
ECLII buffer for chemiluminescent detection of HRP-conjugated antibodies
-
Alternatively, use Clarity Western ECL Substrate A and Substrate B (Bio-Rad, 170-5061) instead of the ECL buffers.
Embryo buffer for testing antibodies (EB)
Gel-loading solution for protein samples (select one of the following; see Step 6)
Phosphate buffered saline (PBS; Sigma-Aldrich P4417-50TAB [1 tablet/200 mL])
Protease inhibitor cocktail tablets, cOmplete EDTA-free (Roche)
SDS-PAGE gel and running buffer (see Step 7)
Tris-buffered saline with detergent (TBSTw) for western blotting
Western blotting transfer buffer
Xenopus embryos (sets of 10 in microcentrifuge tubes)
-
Refer to advice at Xenbase.org for collecting the embryos, the correct stage of development to use for the test can be predicted using available stage-specific transcriptomes (Owens et al. 2016). Caution is needed, however, because differences between protein and mRNA levels exist in embryos (Peshkin et al. 2015; Sun et al. 2014). A positive control protein, often the immunogen, is thus recommended on the blot, and using extract from multiple developmental stages can also mitigate uncertain expression patterns.
Equipment
Heating block at 95°C
ImageQuant LAS4000 or equivalent
Microcentrifuge (at room temperature or at 4°C; see Step 4)
Microcentrifuge tubes
Micropipette with 200-µL tip (see Step 3)
Nitrocellulose membrane
Rocker
SDS-PAGE equipment (see Step 7)
Vortex
Western blotting equipment (see Steps 8–10)
Whatman 3 MM paper or equivalent
METHOD
-
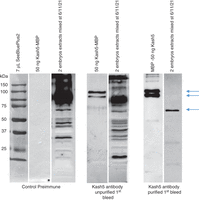
Detailed western blotting protocols are available (e.g., Mahmood and Yang 2012). The advantages of including a positive control antigen and purifying antibodies are clearly seen in Figure 1. It is difficult to identify the additional band in the immune serum before purification but nonetheless the response is clear (compare the antigen lanes in the preimmune and immune samples).
Western blot to test a purified Kash5 antibody. The three gels were run and treated under the same conditions, the only variable being the primary antibody used. Proteins from embryos at stages 6, 11, and 21 were isolated and run using extracts from two embryos per lane. The positive control, an MBP-Kash5 protein expressed in E.coli (calculated MW approximately 90 kDa) runs as two major bands at 90–95 kDa (upper arrows) while the native Kash5 from embryo extract runs as a single band at 59 kDa (lower arrow). The latter is only detected as a unique, clear band once the immune serum has been purified using the unpurified and purified forms of the antibody. MBP-Kash5 is not seen when the preimmune serum was used. The high background levels observed before purification of the antibody were minimized and Kash5 (bottom arrow at 59 kDa) was detected as a single protein of the correct size once the antibody was purified.
Embryo Extract Preparation
-
1. Remove all buffer from each microcentrifuge tube containing a set of 10 embryos.
-
As a general rule of thumb, use two embryos per lane of the gel.
-
-
2. Add 150 µL of EB supplemented with cOmplete EDTA-free protease inhibitor cocktail tablets to each set of 10 embryos.
-
3. Manually disrupt the embryos by pipetting them up and down with 200-µL tip until the solution is homogeneous.
-
At this point, the homogenate can be stored at −80°C if necessary.
-
-
4. Extract the proteins with or without 1,1,1-trichlorotrifluorethane as follows. Keep in mind that yolk solubility may be increased if 1,1,1-trichlorotrifluorethane extraction is omitted.
Extraction with 1,1,1-Trichlorotrifluorethane-
i. Add an equal volume of 1,1,1-trichlorotrifluorethane, and vortex very thoroughly.
-
ii. Centrifuge at 16,000g in a microcentrifuge for 5 min at room temperature.
-
iii. Centrifuge the lysate at 16000g for 30 min at 4°C.
-
iv. Aspirate the lipid layer from the top of the clear lysate.
-
-
5. Pipette the clear top layer (∼200 µL), which contains yolk-depleted protein extract, and place it in a fresh tube.
-
6. Add one of the gel-loading solutions to a final concentration of 1×. Incubate for 3 min at 95°C.
-
7. Immediately separate the proteins by SDS-PAGE using any standard procedure.
-
Alternatively, store the protein extracts at −80°C until use.
-
Membrane Transfer
-
8. Insert the following items into the western blot holder in order:
-
i. a fiber pad,
-
ii. two Whatman 3MM paper pads,
-
iii. the gel,
-
iv. the nitrocellulose membrane,
-
v. two Whatman 3MM paper pads covering the membrane, and
-
vi. another fiber pad.
-
-
9. Place the “sandwich” into the tank, ensuring that the gel is on the negative side, and fill the tank with western transfer buffer.
-
10. Transfer proteins at 30 mA overnight or at 300 mA for 2 h.
Membrane Blocking
-
11. Wash the nitrocellulose membrane with dH2O for 5 min at room temperature.
-
12. Incubate the membrane in 25 mL of the blocking buffer of choice for 1 h at room temperature or overnight at 4°C.
Antibody Incubations
-
13. Replace the blocking buffer with fresh blocking buffer containing the primary antibody at the appropriate dilution. Incubate with gentle agitation for 1–2 h at room temperature or overnight at 4°C.
-
Serial dilutions of the new antibody can be used to determine the appropriate concentration.
-
-
14. Wash the membrane as follows depending on if the primary antibody is unconjugated or conjugated to a label. Conduct all membrane washing steps at room temperature.
For Unconjugated Primary Antibodies-
i. Wash the membrane three times for 5 min each with 10–15 mL of TBSTw.
-
ii. Incubate the membrane with fresh blocking buffer containing the species-appropriate HRP-conjugated secondary antibody according to the manufacturer's recommended dilution with gentle agitation for 1 h.
-
For example, a 1:2000 dilution is used for the Anti-Rabbit IgG-Peroxidase conjugated secondary antibody (Sigma-Aldrich A0545).
-
-
iii. Wash the membrane twice for 5 min each with 15 mL of blocking solution.
-
iv. Wash once for 5 min with TBSTw.
-
v. Rinse once with PBS for 5 min.
-
vi. Wash the membrane twice for 5 min each with 15 mL of blocking solution.
-
vii. Wash the membrane once for 5 min with TBSTw.
-
viii. Rinse once with PBS for 5 min.
-
Protein Detection
-
15. Incubate the membrane at room temperature for 1 min with 10 mL of ECLI buffer and 10 mL of ECLII buffer.
-
Alternatively, incubate the membrane at room temperature for 5 min with 7 mL Clarity Western ECL Substrate Solution A and 7 mL of Clarity Western ECL Substrate Solution B.
-
-
16. Visualize using an ImageQuant LAS4000 or equivalent following the manufacturer's instructions, varying the exposure from 1 sec to 10 min as necessary.
-
See Troubleshooting.
-
TROUBLESHOOTING
Problem (Step 16): The proteins were not fully denatured prior to running the gel, causing bands to be seen at twice or three times the size of the monomer on the membrane.
Solutions: The ability of the proteins to denature under particular conditions varies. Both the loading buffer and denaturing temperature can be optimized. The 2× SDS loading dye or 2× β-mercaptoethanol sample buffer are commonly used. Potential temperature treatments include for 3 min at 95°C, 20 min at 55°C, and the combination of 20 min at 55°C followed by for 3 min 95°C.
Problem (Step 16): The antibody concentration was not optimized, causing the western blot to have high background.
Solutions: To obtain a clear and low-background western blot, optimize the antibody concentration as follows.
-
1. Adjust the gel's comb so that there are only two lanes, a single well for the size marker and a joint, long well containing the sample.
-
2. Run the gel and transfer as normal.
-
3. After transfer, cut the membrane containing the sample proteins into strips, leaving the first strip to contain the size marker and some of the sample.
-
4. Incubate the first strip with the preimmune serum.
-
5. Incubate the rest of the strips under the same conditions but with varying antibody concentrations.
Problem (Step 16): The antibody stability was poor, resulting in western blot results that were unclear or not reproducible.
Solutions: Avoid freeze-and-thaw cycles. Store the antibody lyophilized at −20°C for long-term storage (years) and at 4°C for short-term storage (weeks/months). Purified antibodies tend to be more stable than the raw serum; therefore, purification of serum is suggested if results with raw serum vary.
DISCUSSION
To determine whether an immunized animal has produced antibodies against an antigen, western blotting (Mahmood and Yang 2012) or an ELISA (Hornbeck 1992) are performed. Further information on the effectiveness of the antibody is then obtained by determining fixation conditions on cultured cells expressing the antigen. The final test of the antibody is by immunohistochemistry on embryos or tissue sections (see Protocol: Whole-Mount Fluorescence Immunocytochemistry on Xenopus Embryos [Lee et al. 2008]). Detected protein size is not provided by the ELISA, so western blotting, which has been made more quantitative with fluorescent secondary antibodies and fluorescence readers, is described here.