Screening of Chemical Libraries Using Xenopus Embryos and Tadpoles for Phenotypic Drug Discovery
- 1Walter-Brendel-Center of Experimental Medicine, University Hospital, Ludwig-Maximilians-University Munich, 81377 Munich, Germany
- ↵6Correspondence: abrandli{at}med.lmu.de
Abstract
Phenotypic drug discovery assesses the effect of small molecules on the phenotype of cells, tissues, or whole organisms without a priori knowledge of the target or pathway. Using vertebrate embryos instead of cell-based assays has the advantage that the screening of small molecules occurs in the context of the complex biology and physiology of the whole organism. Fish and amphibians are the only classes of vertebrates with free-living larvae amenable to high-throughput drug screening in multiwell dishes. For both animal classes, particularly zebrafish and Xenopus, husbandry requirements are straightforward, embryos can be obtained in large numbers, and they develop ex utero so their development can be monitored easily with a dissecting microscope. At 350 million years, the evolutionary distance between amphibians and humans is significantly shorter than that between fish and humans, which is estimated at 450 million years. This increases the likelihood that drugs discovered by screening in amphibian embryos will be active in humans. Here, we describe the basic protocol for the medium- to high-throughput screening of chemical libraries using embryos of the African clawed frog Xenopus laevis. Bioactive compounds are identified by observing phenotypic changes in whole embryos and tadpoles. In addition to the discovery of compounds with novel bioactivities, the phenotypic screening protocol also allows for the identification of compounds with in vivo toxicity, eliminating early hits that are poor drug candidates. We also highlight important considerations for designing chemical screens, choosing chemical libraries, and performing secondary screens using whole mount in situ hybridization or immunostaining.
MATERIALS
Reagents
-
Prepare all solutions in sterile, deionized, milliQ-filtered H2O.
Chemical library collections (see Table 1 for examples)
Selection of commercial chemical libraries
Compound E stock solution (2 mm)
Dimethyl sulfoxide (DMSO) (Sigma-Aldrich 154938; purity 99%)
l-cysteine solution (2.0% w/v)
Marc's Modified Ringer solution (MMR) (10× stock)
-
Dilute to 0.1× with H2O before use.
Phosphate-buffered saline (PBS)
-
Adjust pH to 7.4.
Tricaine (0.5 mg/mL in 0.1× MMR)
Xenopus laevis embryos
-
Obtain wild-type or albino embryos by in vitro fertilization using sexually mature Xenopus laevis males and females. Alternatively, embryos may also be obtained from natural matings (see Protocol: Obtaining Xenopus laevis Embryos [Shaidani et al. 2021]). Where appropriate, embryos from genetically modified Xenopus strains or embryonic disease models generated by antisense-morpholino oligonucleotide knockdown can be used. Embryos and tadpoles are staged according to Nieuwkoop and Faber (NF) (Nieuwkoop and Faber 1994).
Equipment
Aluminum foil
Cell culture incubator (18°C–23°C; humidified)
Cell culture plates, 48-well clear flat bottom, TC-treated, polystyrene (Corning Falcon 353078)
Dumont #5 forceps (stainless steel; Sigma-Aldrich)
Fume hood
Microcentrifuge tubes (1.5-mL)
Micropipettes (P20, P200, P1000) with filter tips of appropriate sizes
Multichannel pipette or liquid handling robot (e.g., Aquarius, Tecan) (see Step 1)
Nutator (Clay Adams BD 421125)
Parafilm M wrapping film (Fisher Scientific)
Petri dishes (6-cm, 10-cm, uncoated plastic)
Plastic plates, 96-well, clear round-bottom, polystyrene (Corning 3788)
Plastic wrap (e.g., Saran Wrap)
Stereomicroscope (e.g., Carl Zeiss SV6) with two-armed fiber optic illumination
-
This microscope is used for embryo handling and phenotype scoring.
Stereomicroscope (e.g., Leica M205 FA) equipped with a digital camera
-
This microscope is used for imaging of embryos.
Transfer pipettes (5-mL, plastic, disposable)
METHOD
-
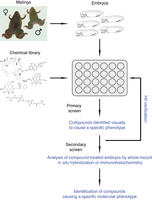
An overview of the general strategy for phenotypic drug discovery using Xenopus embryos is shown in Figure 1. The method described below is a protocol for the primary screen. Options for secondary screens are described subsequently.
-
Adhere to local IACUC regulations regarding animal handling.
High-throughput phenotypic drug discovery using Xenopus embryos. The flowchart shows the steps involved in phenotypic chemical screening. Once embryos have reached the desired embryonic stage, they are arrayed in 48-well plates and treated with single compounds taken from the chemical library of choice. In the primary screen, embryos are scored visually by bright-field microscopy for the desired developmental phenotype(s) or morphological change(s). Positive hits are verified by repeating the primary chemical screen. In the secondary screen, embryos are treated with verified compounds and subsequently analyzed by whole-mount in situ hybridization or immunohistochemistry. Figure adapted from Kälin et al. (2009).
Chemical Library Handling
-
1. Dilute stock compounds of the chemical library to a concentration of 2 mm in DMSO (50 µL volume). Transfer aliquots of the chemicals from the mother plates to the daughter plates containing DMSO using a liquid handling robot or a multichannel pipette.
-
Most compounds of commercial chemical libraries are predissolved in DMSO at a concentration of 10 mm or occasionally at 2 mm. Note that depending on the library, some compounds might be dissolved in H2O instead of DMSO.
-
-
2. Store diluted stock plates at −20°C (2 yr) or −80°C (5 yr).
Embryo Staging, Selection, and Distribution
-
3. Once in vitro fertilized Xenopus eggs have reached the two- or four-cell stages, remove the jelly coats of the embryos by gentle swirling in 2% l-cysteine solution at room temperature for 3–4 min using a nutator until the jelly coats are dissolved. Rinse embryos with 0.1× MMR solution at room temperature until no cysteine odor can be detected.
-
4. Divide the embryos using Dumont #5 forceps into batches of comparable embryonic stages. Use plastic pipettes to transfer them into several large 10 cm Petri dishes containing 0.1× MMR. Avoid overcrowding by pooling fewer than 100 embryos per dish.
-
5. Culture embryos at 23°C in a humidified incubator until they reach the desired embryonic stage to initiate compound testing. Pool embryos of the same embryonic stage.
-
The culture temperature can range between 18°C and 23°C. The lower the temperature, the slower embryonic development proceeds.
-
See the subsection Timing and Duration of Exposure to Chemical Compounds below for guidance regarding timing.
-
See Troubleshooting.
-
-
6. Inspect embryos with a stereomicroscope. Use a plastic transfer pipette to remove and dispose of any abnormal (i.e., unfertilized, necrotic, or misshapen) embryos. Repeat the selection process again later, if necessary.
-
This step guarantees that only embryos of highest quality will be used for chemical screening.
-
Chemical Library Screening
-
7. Aliquot 1 mL screening medium to each well of a plastic 48-well dish.
-
8. Use a plastic transfer pipette to place five embryos of the desired embryonic stage into each well.
-
For consistent results, the embryos should not be younger than late blastula or early gastrula stages. With multiple embryos per well, the penetrance of a compound-induced phenotype can be assessed. Five embryos per well are optimal.
-
-
9. To start compound screening, add 10 µL aliquots of the diluted chemicals to the wells using a P20 micropipette with filter tips, resulting in a final concentration of 20 µm for each compound. Consider the following controls: embryos treated with screening solution only (negative control) and embryos treated with a reference compound that is known to induce a specific phenotype (positive control). To guarantee equal screening conditions, include controls in each 48-well dish.
-
Compounds that elicit a phenotype will be retested at concentrations that may range between 1 µm and 50 µm. If you are screening larger chemical libraries (more than 100 compounds), performing the initial screen at 20 µm is a good starting concentration. The γ-secretase inhibitor compound E (20 µm) can serve as a positive, edema-inducing reference compound (Kälin et al. 2009).
-
See Troubleshooting.
-
-
10. Incubate embryos in a humidified incubator at 23°C until they have reached the desired end point. Typically, the time frame is between 1 and 5 d.
-
Add H2O to each well if fluid evaporation is observed. Do not grow embryos beyond stage 45 in 48-well dishes as they become too large (>10 mm).
-
See Troubleshooting.
-
Scoring of Phenotypes
-
11. Score any visible phenotypes of the embryos every 24 h using a stereomicroscope. Record embryonic mortality in each well. Remove dead embryos using a plastic transfer pipette and discard. Use a new transfer pipette each time to avoid contaminating wells with compounds.
-
See the subsection Types of Phenotypes below for details on phenotypes.
-
Chemicals are considered to be active when at least 80% (four out of five embryos) display the same phenotype (i.e., edema, lethality, or other phenotypes). Independent phenotype scoring by multiple investigators is useful to reduce false positives or to avoid missing subtle phenotypes.
-
See Troubleshooting.
-
Imaging
-
12. At the end of the drug screening assay, anesthetize Xenopus embryos in tricaine (0.5 mg/mL) in 0.1× MMR for at least 5 min at room temperature.
-
13. Capture images of anesthetized embryos in solution using a stereomicroscope equipped with a high-resolution digital camera. Anesthetized embryos can be imaged either in the 48-well dishes or after transfer to 6-cm Petri dishes.
Fixing Embryos for Storage
-
14. After imaging, remove 0.1× MMR solution and fix Xenopus embryos by adding 1 mL of 1× MEMFA to each well of the 48-well dish. Incubate embryos in a fume hood for 2 h at room temperature.
-
Make sure the embryos are completely covered with MEMFA.
-
-
15. Remove the fixative. Wash the embryos with 1× PBS three times for 15 min each at room temperature using a nutator.
-
16. Store embryos in 1× PBS at 4°C in the 48-well dishes. Seal dishes with plastic wrap to prevent evaporation.
-
Fixed embryos may be used for further analysis by in situ hybridization or immunohistochemical staining.
-
Confirmatory Testing
-
17. Retest all bioactive compounds that have elicited specific phenotypes to eliminate “false positive” hits. A bioactive compound should modulate a biological process in a dose-dependent manner. Therefore, validate the top hits at multiple concentrations. For example, if the screen was performed at 20 µm, perform a dose curve starting at 80 µm with twofold serial dilution down to 1.25 µm.
-
It is also advisable that all hit compounds of interest are repurchased or resynthesized and retested in the original screening assay. Besides weeding out false positives, this will confirm the identity of the compound in the chemical library stock plate.
-
Detection of Phenotypes
-
18. Observe compound-induced morphological phenotypes by direct observation with a stereomicroscope.
-
The human eye is a superb tool for detecting even subtle morphological changes or altered behavior of treated embryos. An experienced researcher will screen several dozen 48-well plates per day, which amounts to well over 1000 compounds per day. This enables moderate phenotypic screening campaigns using chemical libraries comprised of a few thousand compounds to be performed in a matter of weeks.
-
See the Discussion for information on types of phenotypes and automated imaging systems.
-
TROUBLESHOOTING
Problem (Step 5): Embryos are heterogeneous in age.
Solution: Heterogenous embryos can be caused by overcrowding of collected embryos, sick embryos, or poor clutch quality. These problems can be potentially remedied by placing <100 embryos per 10-cm Petri dish, removing all dying or misshaped embryos before plating, and using alternative parents to generate embryos.
Problem (Step 9): The chemical library is too large to be screened by a single investigator.
Solution: An investigator can usually handle the screening of up to 200 compounds per day. If the library is too large to screen in a single day, screen embryos with pools of five compounds and then repeat the screen with single compounds for pools testing positive.
Problem (Step 10): Embryos appear to be drying out.
Solution: This can occur because of excess evaporation of the screening medium. To prevent evaporation, check embryos regularly and add H2O to wells to compensate for evaporation. Alternatively, seal a 48-well plate with Parafilm M wrapping film.
Problem (Step 10): Embryos die during incubation with compounds.
Solution: Embryos may die for any of the following reasons: toxicity of the test compound, the pH of the screening solution deviating from pH 7.4–7.5, treatment of embryos with compounds too early in embryogenesis, poor embryo quality leading to premature death, lack of food, or large size of embryos. Potential solutions are:
-
1. The chemical in question may be known to cause embryonic lethality; try reducing compound concentrations.
-
2. Adjust the pH of screening solution to 7.5.
-
3. Use older embryos, if possible.
-
4. Remove dying embryos immediately to protect the remaining embryos in the well; if untreated control embryos are dying, repeat the chemical screen with embryos obtained from alternative parents.
-
5. The 48-well format for drug screening is optimal for embryos up to about stage 45. If your end point requires older embryos, adapt the screening protocol for 24-well plates. Keep in mind that older embryos will require feeding, which will complicate chemical screening.
Problem (Step 11): The phenotype is weak and affects only a minority of embryos.
Solution: A weak phenotype may occur because the compound concentration is too low to detect a fully penetrant phenotype (dosing effect). Another possible reason is that embryos were treated either too early or too late in embryogenesis. Solutions to these problems are to retest the compounds at higher concentrations and/or to start exposing embryos either earlier or later to the compound.
DISCUSSION
The use of Xenopus embryos as an alternative to zebrafish larvae for whole organism–based phenotypic drug discovery screening was first proposed in 2004 (Brändli 2004) followed by a small-scale study of 14 compounds demonstrating that they induced a range of morphological phenotypes in Xenopus embryos (Tomlinson et al. 2005). Subsequent chemical screens were designed to identify compounds inducing a specific phenotype, altering morphology, or modifying organ function(s) (Kälin et al. 2009; Tomlinson et al. 2009; Dush et al. 2011; Tanaka et al. 2016; Willsey et al. 2021). Although chemical screens vary in their phenotypic readouts, certain steps are shared across different strategies. Here, we would like to highlight some general considerations when designing chemical screens using Xenopus embryos and tadpoles.
Design of a Chemical Screen
Central to any chemical screening strategy is that the anticipated phenotypes caused by bioactive compounds should be specifically linked to the biology or physiology of the targeted tissue or organ of the Xenopus embryo or tadpole. Hence, the aims and readouts of a chemical screen using whole organisms must be considered carefully. What is the developmental process that should be modulated or altered by treatment with a small molecule? Which biochemical pathway(s) should be inhibited or modulated? Is the anticipated morphological readout predictive of the underlying biochemical pathway? Is the screen supposed to be broad, targeting development of an entire organ, or more narrowly aimed at specific signaling molecules or pathways modulating morphogenesis or organ physiology? It is also important to consider whether the end point of the chemical screen leads to (1) a phenotype that can be easily scored by bright-field imaging, (2) a phenotype that requires the detection of a specific marker (i.e., mRNA or protein by in situ hybridization or immunohistochemistry), or (3) a behavioral phenotype. In general, the ideal readout of the chemical screen should be rapid, sensitive, accurate, and reliable to detect the desired modulation of a biological, developmental, or physiological process in vivo. Every phenotypic readout has its advantages and disadvantages. Phenotype detection by visual inspection is rapid, can be performed multiple time over several days, and does not require time-consuming tissue processing. In addition, the human eye is superb at picking up both subtle and complex phenotypes. However, internal morphological alterations are usually not detected. Whole-mount in situ hybridization and immunohistochemical staining can reveal changes in the spatial expression of marker genes at high resolution and can be considered semiquantitative. However, these procedures require fixing embryos and thus terminating the chemical screen. Furthermore, they are time-consuming, taking at least 3 d to complete. Finally, transgenic Xenopus tissue- or pathway-specific reporter lines are convenient for fluorescence imaging–based screening, particularly for assaying alteration of internal organs. However, generating a stable transgenic line requires access to suitable promoters to drive reporter gene expression and can easily take one to two years.
Selection of a Chemical Library
The ideal chemical library possesses as much chemical diversity as possible and a high proportion of compounds with drug-like physicochemical parameters. A large selection of chemical libraries is available from a variety of commercial vendors and the majority of chemical screens performed in zebrafish and Xenopus have used these libraries. They range from large libraries consisting of thousands of compounds to small collections of compounds consisting of inhibitors or targeting specific pharmacological classes or biochemical pathways (Table 1). When choosing a chemical library for screening with Xenopus embryos, it is important to consider the level of characterization of compounds in the library and whether it is possible to purchase single chemicals for retesting and validation. Using libraries containing FDA-approved drugs or known bioactive compounds has the advantage that toxicological and mechanistic information is readily available. However, using these libraries is biased toward less novel mechanisms and may yield compounds which are patent-protected, limiting their development as potential therapeutics. Commercial libraries have an important advantage over in-house synthetic libraries. The former typically consist of compounds that are drug-like, adhering to Lipinski's rules (Lipinski et al. 2001). Critical factors include the compound's molecular weight, hydrophobicity, and number of hydrogen bond donors and acceptors (Lipinski et al. 2001). A further critical parameter is the logarithm of the partition ratio between octanol and water (log P). The log P value of a compound correlates well with its membrane permeability. Generally, compounds with log P values greater than +1 are absorbed well by zebrafish and Xenopus embryos (Wheeler and Brändli 2009). Low molecular weights, typically 200–700 Da, and favorable partition coefficient values afford good drug penetration, pharmacokinetics, and dynamics. On the other hand, synthetic libraries have the advantage that they may contain small molecules with unique properties and novel pharmacological activities. These libraries are therefore suitable for identifying new functional chemicals and biological targets. However, once discovered, these novel compounds may require significant efforts to determine their mechanisms of action. Unfortunately, many commercial synthetic libraries consist of compounds that are easy to synthesize or are biased toward a few simple core structures rather than a broad array of diverse compounds. Public repositories such as those of the U.S. National Institutes of Health (https://ncats.nih.gov/preclinical/core/compound) or the Drug Repurposing Hub of the Broad Institute (https://clue.io/repurposing) offer attractive alternatives to commercial chemical libraries. Similar to commercial sources, public repositories offer access to curated and annotated collections of U.S. Food and Drug Administration (FDA)-approved drugs, clinical trial drugs, and preclinical tool compounds with companion information resources. Prior to embarking on a screening campaign, it is, however, important to inquire about the availability of individual compounds for validation and retesting purposes.
Targeted chemical screens with small libraries of compounds of known functionality are also powerful. These libraries are composed of compounds targeting a specific type of biochemical activity, such as kinases, neurotransmitters, or G-protein-coupled receptors. Using this screening approach can uncover previously unknown biochemical pathways regulating a developmental process in Xenopus embryos or tadpoles. Ultimately, the choice of the optimal chemical library is highly dependent on the type of screen and the aim of the chemical screen.
Choice of Animals to Be Treated
Most chemical screens are performed using wild-type embryos or tadpoles, which are obtained from different clutches to ensure consistent phenotype penetrance and reduce the impact of genetic variability. Wild-type screens are straightforward given that wild-type frogs are readily available and the embryos do not require genotyping before chemical screening. In phenotypic screens that could be obstructed by the development of pigment cells, the use of unpigmented embryos from the mating of albino frogs should be considered. If chemically induced alterations in internal organs are the aim of the investigation, it is best to use embryos obtained from transgenic Xenopus lines expressing fluorescent reporter proteins. The availability of suitable Xenopus reporter lines presently represents a major limitation for these types of chemical screens. Finally, Xenopus models of human diseases generated either by knocking down gene functions using morpholino antisense oligonucleotides or by CRISPR–Cas-mediated gene editing represent attractive starting points for chemical screening and phenotypic drug discovery (Schmitt et al. 2014). In these cases, the aim of the chemical screening would be to identify compounds able to suppress or ameliorate the disease phenotype in vivo using mutant embryos or tadpoles. Paramount for this approach to work is that the Xenopus disease model has to recapitulate the key pathophysiological hallmarks of the human disease.
Timing and Duration of Exposure to Chemical Compounds
Appropriate timing of compound addition depends on the biological process to be modulated. Generally, exposure of embryos or tadpoles to the compounds should start before and continue until the process of interest is completed. Nieuwkoop and Faber's Normal Table of Xenopus laevis provides detailed information on the timing and development of various tissues and organs during Xenopus development (Nieuwkoop and Faber 1994). In addition, Xenbase is a valuable online resource that integrates diverse biological, gene expression, and phenotypic data and should be consulted before settling on the timing parameters of chemical screens. Toxicity is a further factor to keep in mind. Extended exposures to chemicals increase the death rates of embryos compared to the negative control embryos. Furthermore, younger embryos are more vulnerable to the harmful effects of some compounds. In our experience, treatment of embryos before they have reached the gastrulation stage significantly increases the death rates of embryos compared to the negative controls. Additionally, prolonged compound exposure can reveal teratogenicity or cytotoxicity of test compounds. It is therefore advisable to go for extended compound exposure times in the initial screening campaign, which is typically performed at one fixed final concentration. Compounds found to be toxic can subsequently be retested at lower doses. A washout step to remove the chemicals can be considered if there is a possibility that the drug could interfere with a later essential developmental step that is not the aim of the chemical screen or if the readout of the screen (e.g., an enzymatic reporter gene assay) could be affected by certain compounds present in the chemical library.
Anticipated Results
Toxicity
The general toxicity of chemical library compounds depends on (1) final compound concentration, and (2) the embryonic stage used at first exposure. Ideally, each compound would be screened using a range of concentrations (e.g., from 0.1 to 100 µm). However, this is not practical when larger libraries containing thousands of compounds are used for chemical screening. For the initial screen, a single final compound concentration of 10 or 20 µm has been found by many laboratories to be a good compromise. Regarding general compound toxicities, younger embryonic stages, such as pregastrula stages, are more sensitive than older ones. The toxicity rate (i.e., the percentage of compounds causing embryonic lethality) is also dependent on the type of chemical library used for screening. Chemical libraries comprised of FDA-approved drugs or known bioactive compounds, such as the LOPAC1280 library (see Table 1), contain compounds that have been subjected to toxicity assessments. These libraries usually have lower toxicity rates than synthetic compound libraries. We screened the LOPAC1280 library for bioactive compounds using Xenopus late tailbud stage embryos (stage 31, 37 h postfertilization) and found that 4% of the compounds caused either embryonic or larval lethality (Kälin et al. 2009). Similar toxicity rates were reported in chemical screens using zebrafish (Kaufman et al. 2009).
Hit Rates
Hit rates are the percentage of compounds that elicit the predefined “desired” phenotype, and they are highly variable, ranging from the lower single digits down to 0%. Multiple parameters, including dosing, treatment time point, and length of compound exposure, influence the observed hit rates. In general, lower hit rates are a more promising outcome of a chemical screen than high rates, which could indicate a general problem in the chemical screening strategy or a lack of specificity of the compounds at the dose(s) tested. Furthermore, hit rates vary significantly between different chemical libraries. Screens using libraries of known bioactive compounds usually result in higher hit rates than those relying on libraries of synthetic compounds with unknown pharmacology. Generally, screening concentrations of 10 to 30 µm produces acceptable hit rates. With a compound concentration of 20 µm, we observed a hit rate of 3.7% in a screen of the LOPAC1280 library for compounds capable of inducing edema formation in Xenopus tadpoles (Kälin et al. 2009). Others reported hit rates of 1.4% and 2.2% depending on the chemical library used (Tomlinson et al. 2009). Generally, a hit rate of 1%–3% is reasonable to expect.
Detection of Phenotypes with Automated Systems
Various automated imaging and motion-tracking systems are commercially available. They are, however, costly and time-consuming to implement. Automated morphological screens are also complicated by the fact that Xenopus embryos change orientation once they start moving. Movements develop from simple local twitching of a few swimming muscles starting at stage 37/38 to free forward-moving, upright swimming by stage 45 (Muntz 1975). Customized imaging systems can track phenotypes in moving embryos and tadpoles; however, we only recommended them in the following cases: (a) when chemical library screening becomes a core activity of the laboratory; (b) when the goal is to screen chemical libraries larger than 10,000 compounds; or (c) when performing complex behavioral screens (see the subsection Behavioral Readouts below).
Types of Phenotypes
There are many phenotypes that can be scored by observation of wild-type Xenopus embryos and tadpoles after compound treatment. Two general types of phenotypes, morphological and behavioral alterations, can be scored by eye. Chemical screens can be designed to identify compounds that induce a specific change in morphology. Multiple traits, such as alterations in body axis, eye shape, skin pigmentation, or heartbeat, can be easily examined. Additionally, an increasing number of transgenic lines expressing tissue-specific fluorescent reporter proteins are being developed in the Xenopus community (see https://www.xenbase.org). These can be used to conveniently examine compound-induced changes in internal organs, such as the heart, pronephric kidney, and blood vessels. The aim of such experiments is to generate a hypothesis about biochemical pathways underlying a developmental process. The discovery of such chemical modifiers could also be useful if they can be correlated with particular disease pathways.
Morphological and Developmental Phenotypes
A variety of morphological and developmental phenotypes caused by chemical perturbation have been characterized. For example, alterations in pigment cell development and migration are easily scored by visual inspection. High-throughput screens with Xenopus embryos led to the identification of several compounds that disrupt pigment cell development (Tomlinson et al. 2009). Interestingly, one of these compounds is structurally related to lefunomide, which is now being developed as potent inhibitor of melanoma growth (White et al. 2011). Similarly, anticancer drug candidates were identified by screening for compounds that perturb gastrulation or neural crest migration in vivo (Tanaka et al. 2016). Heterotaxia (reversal organ laterality) is a further morphological feature that can be easily scored by examining the looping of the heart and gut in compound-treated embryos. A small molecule screen for left–right asymmetry in Xenopus embryos identified a pyridine analog named heterotaxin. This compound disrupts both heart and digestive organ laterality and acts by inhibiting TGF-ß signaling (Dush et al. 2011). Pathophysiological phenotypes, such as edema formation, can also serve as a starting point for a chemical screen with Xenopus embryos. Edema or fluid-filled swellings can be used as convenient indicators of impaired cardiovascular, lymphatic, and/or excretory system functions. Using edema formation as the primary end point in a small-molecule screen, we identified several compounds regulating lymphatic and blood vascular development (Kälin et al. 2009). Importantly, an adenosine A1 receptor antagonist was also able to block neovascularization in adult mice. In a final example, an oncology drug screen using Xenopus tropicalis embryos identified 17 compounds that enhance or suppress neural progenitor cell proliferation in vivo (Willsey et al. 2021). Three of these compounds were involved in estrogen signaling. Given that alterations of cortical neurogenesis have been implicated in the vulnerability to autism spectrum disorders, estrogen might mitigate the effects of disparate autism gene mutations.
Behavioral Readouts
Beyond morphology, behavioral readouts can be also used for chemical screens in whole organisms. There are numerous physiological and behavioral phenotypes that can be assessed. For example, changes in the movement or swimming pattern can be scored in response to compound treatment. Compounds altering behavior in Xenopus embryos or tadpoles frequently elicit their activity by acting on the central nervous system. This in turn offers the opportunity for the discovery of novel neuroactive drugs, which is impossible using in vitro systems as they cannot recapitulate the biology and neurophysiology of an entire organism. Large-scale behavioral screens using Xenopus embryos have to date not been reported. In zebrafish, screens were performed to identify small molecules that alter acute photometer response or interfere with rest and wakefulness patterns (Kokel et al. 2010; Rihel et al. 2010; Chiu et al. 2016). Others have screened for compounds interfering with habituation to acoustic startle (Wolman et al. 2011) or convulsive and electrographic seizures (Baraban et al. 2013). Similar screens are also possible with Xenopus embryos. Given that the evolutionary distance to humans is significantly shorter for amphibians than fish, therapeutic translation of bioactive compounds might be more straightforward (Wheeler and Brandli 2009). Seizures characterized by uncontrolled tail bends and excessive turning can be induced in tadpoles by bath application of several common chemoconvulsants including pentylenetetrazole (PTZ), a GABAA antagonist (Hewapathirane et al. 2008). Using a Xenopus tadpole model of PTZ-induced epilepsy, a previously unknown neuroprotective role of polyamines was discovered (Bell et al. 2011). Xenopus tadpoles have also long been used in anesthesiology research (Downes and Courogen 1996). Two forms of behavioral assessments, loss of spontaneous swimming movements and the loss of elicited movement, are consistent and simple end points to assess anesthetic potency with Xenopus tadpoles. Importantly, the relative EC50 values for most anesthetics are strikingly similar between Xenopus and mammals, including humans (Woll and Eckenhoff 2018). Hence, tadpole bioassays are a cost-effective way to determine relative drug tolerance and cross-tolerance before entering more expensive testing in mammalian animal models. In addition, high-throughput screening methods for nonvolatile and volatile anesthetics using Xenopus tadpoles have been recently described (Woll and Eckenhoff 2018). Irrespective of the type of behavioral assay used, behavioral screens are ideally performed using automated imaging and motor activity tracking, because complex phenotypes are difficult to follow by eye. In addition, measuring, quantitation and statistical analysis can be automated using commercial motion-tracking systems. Overall, the potential of employing Xenopus embryos and tadpoles in behavioral assays to identify neuroactive and psychotropic drugs has to date not been fully tapped.
Secondary Screening
Depending on the nature of the primary phenotypic screen, a secondary screen may be necessary to determine those compounds affecting the process of interest or to further confirm the bioactivity of the compounds. Examples include subtle morphological phenotypes, particularly those affecting internal organs, changes in mRNA levels, or protein modifications, such as phosphorylation or degradation, which are not evident to the naked eye. Secondary assay methods rely either on in situ hybridization, immunohistochemistry, or reporter protein expression using transgenic lines. In situ hybridization uses an antisense mRNA probe to determine the spatial expression of a specific mRNA in fixed embryos or tadpoles. A color reaction linked to the antisense mRNA probe localizes expressed transcripts to specific tissues and organs. Immunohistochemistry is used to identify the expression levels of a specific protein or post-translational modification via specific antibodies. Finally, it is possible to screen transgenic lines expressing fluorescent reporters, such as the green fluorescent protein, in a tissue-specific manner. Detailed protocols for whole mount in situ hybridization and immunostaining of Xenopus embryos and tadpoles have been published elsewhere (see Protocol: Whole-Mount RNA In Situ Hybridization and Immunofluorescence of Xenopus Embryos and Tadpoles [Willsey 2021], Protocol: Whole-Mount Immunocytochemistry in Xenopus [Klymkowsky 2018], and Protocol: Whole-Mount In Situ Hybridization of Xenopus Embryos [Saint-Jeannet 2017]). An example of a two-step chemical screening strategy involving whole-mount hybridization was reported by us in the past (Kälin et al. 2009). The aim of the screen was to identify novel compounds modulating angiogenesis and/or lymphangiogenesis in Xenopus tadpoles. The first step involved a simple phenotypic read-out (edema formation or larval lethality) to select 66 bioactive compounds from a 1280-compound library. This was followed by semiautomated in situ hybridization analysis using vascular and lymphatic marker genes to identify 32 compounds that could interfere with blood vascular or lymphatic development, respectively. In situ hybridization allowed for a detailed classification of bioactive compounds by the type of vascular and lymphatic phenotypes detected, including defective vasculogenesis or angiogenesis, ectopic angiogenic sprouting, and defective lymph angiogenesis. Other examples of Xenopus chemical screens that rely on secondary screens with whole mount in situ hybridization and/or immunohistochemistry can be found here (Dush et al. 2011; Tanaka et al. 2016).
Concluding Remarks
Chemical screening using Xenopus embryos and tadpoles has become a very useful approach for the discovery of bioactive compounds and drug-like molecules with the potential of becoming therapeutic agents in the future. This is illustrated best by the discovery of novel compounds that interfere with angiogenesis or lymph vessel development in vivo (Kälin et al. 2009) and drug candidates with anticancer activities (White et al. 2011). Phenotypic drug discovery using Xenopus embryos can be performed in a medium- to high-throughput manner by screening chemical libraries consisting of thousands of compounds. In vivo testing allows for the study of the effects of compounds in a complex biological system. In addition, the toxicity of compounds is simultaneously assessed and compounds with adverse effects can be eliminated early from the drug discovery process. Finally, the emergence of gene editing technologies paves the way for the development of more sophisticated genetic Xenopus disease models in the coming years. It therefore is anticipated that chemical screening using Xenopus embryos and tadpoles will become an indispensable tool in therapeutic drug discovery.
ACKNOWLEDGMENTS
We are grateful to Sabine D'Avis for excellent technical assistance. The work was supported by funds from the University Hospital Munich and in part by a grant of the European Commission (EU FP7 Program, EuRenOmics Grant Agreement 305608) to A.W.B.
Competing interests: A.W.B. and R.E.K. hold U.S. and European patents related to chemical screening in amphibians. The other authors declare no competing interests.
Footnotes
-
↵2 Present address: Sartorius CellGenix GmbH, Freiburg, Germany
-
↵3 Present address: Merck KGaA, Darmstadt, Germany
-
↵4 Present address: Neurosurgical Research, Department of Neurosurgery, University Hospital, Ludwig-Maximilians-University Munich, Munich, Germany
-
↵5 These authors contributed equally to this work.
-
From the Xenopus collection, edited by Hazel L. Sive.