Techniques for Identifying and Sorting Transgenic Mosquito Larvae
- CNRS UPR9022, INSERM U1257, Institut de Biologie Moléculaire et Cellulaire, Université de Strasbourg, 67000 Strasbourg, France
- ↵1Correspondence: e.marois{at}unistra.fr
Abstract
Transgenic mosquitoes are used in many aspects of mosquito research. First, they can help answer biological questions to advance scientific knowledge—for example, in the fields of mosquito–pathogen interactions, insect immunity, or olfaction. Second, transgenic technologies may be used to develop much needed novel vector control strategies, such as mosquitoes that are unable to transmit disease or transgenes that sterilize mosquito females to suppress vector populations. Here, we introduce how researchers use various selection markers to screen for transgenic mosquito larvae following a transgenesis experiment. Common procedures include using a binocular fluorescence microscope for initial screening. For higher-throughput screening, a flow cytometer known as Complex Object Parametric Analyzer and Sorter (COPAS) can be used to stabilize transgenic lines through the purification of homozygous individuals or to manage transgene frequency in established transgenic lines. In particular, COPAS sorting allows the production of mosquito larval cultures composed of a mixture of genotypes (control and genetically modified larvae) with the goal of raising both groups of mosquitoes under the same environmental conditions in preparation for a controlled phenotype assessment. It can also be used to produce large populations of male mosquitoes, which should facilitate the development of mosquito control intervention strategies similar to the sterile insect technique (SIT), which aims to release large numbers of sterile males that will mate with and sterilize wild females to suppress mosquito populations. Finally, the utilization of a puromycin resistance marker cassette to screen for transgenic Anopheles larvae is also introduced.
INTRODUCTION TO TRANSGENIC MOSQUITO PRODUCTION TECHNIQUES
Transgenic mosquitoes have been produced since the late 1990s (Jasinskiene et al. 1998; Catteruccia et al. 2000), mainly by embryo microinjection. Gene gun bombardment is an alternative approach that has yielded transgenic Aedes aegypti (Lule-Chávez et al. 2021). The receptor-mediated ovary transduction of cargo (ReMOT) approach (i.e., ovarian uptake of nucleic acid and protein complexes injected in adult females) has allowed CRISPR–Cas9 editing of marker genes in Aedes and Anopheles (Chaverra-Rodriguez et al. 2018; Macias et al. 2020) and could potentially also be used to insert transgenes in the genome. In all cases, DNA-injected individual mosquitoes must be backcrossed to wild-type mosquitoes, in a cross termed G0, and the G1 progeny, composed of hundreds to tens of thousands of larvae, must be screened to identify transgenic individuals. Depending on the transgenesis approach used (transposon, docking site [which is the integration of foreign DNA into a recombination site previously inserted in the genome], or CRISPR–Cas9-based construct knock-in) and on the technical quality of microinjection, the number of transgenic individuals in a G1 population of 10,000 larvae may range from just a few (e.g., following piggyBac transposon transformation in Anopheles) to a few hundred (CRISPR–Cas9 knock-in of a fluorescent marker gene in Anopheles or piggyBac transposon transformation in Aedes).
The rarity of the desired event underscores the absolute necessity for a robust selection system, in which positive larvae will immediately be distinguishable from wild types without the need for polymerase chain reaction (PCR) genotyping. This is best achieved using fluorescence markers expressed in live mosquitoes, especially during their larval stages, and requires visual examination under a fluorescence microscope as described in an accompanying protocol (Protocol: Screening Mosquito Larvae Under a Fluorescence Binocular Microscope [Marois 2023a]). Alternatively, selection based on resistance to the antibiotic puromycin has been developed for Anopheles larvae and is also described in an accompanying protocol (Protocol: Selecting Transgenic Mosquito Larvae with Puromycin [Marois 2023b]). Once a transgenic line is obtained and amplified, it is often desirable to isolate individuals carrying the transgene in the homozygous state to stabilize the line. Alternatively, it may be decided to maintain the transgenic line as a mix of negative, heterozygous, and homozygous individuals. This ensures that control and transgenic mosquitoes to be compared in subsequent experiments continue to share identical genetic backgrounds and microbiota over time and are grown under the same environmental conditions. When an experiment is planned, animals with the correct homozygous transgenic and control genotypes in equal numbers can be extracted from a population of larvae. For all these purposes, higher-throughput screening techniques are helpful and must be able to distinguish between homozygous and heterozygous individuals based on their fluorescence intensity, which correlates with transgene copy number (two in homozygotes, one in heterozygotes). A flow cytometer–like sorting machine (Complex Object Parametric Analyzer and Sorter [COPAS]) provides high-throughput sorting capacity and is described in Protocol: Sorting Mosquito Larvae with a COPAS Machine (Marois 2023c). The same technique can be applied to the sorting of large quantities of pure male or female larvae, which may facilitate future mosquito control intervention strategies related to the sterile insect technique (SIT) (Lutrat et al. 2019, 2023).
MARKER GENES AND THE CHOICE OF THEIR PROMOTERS IN MOSQUITOES
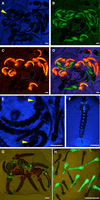
Several marker genes have been validated for the selection of transgenic mosquitoes. The popular fluorescent proteins CFP (cyan fluorescent protein), GFP (green fluorescent protein), YFP (yellow fluorescent protein), and DsRed or mCherry (red fluorescent proteins) and their many derivatives are suitable and frequently used to identify transgenic mosquito larvae and adults. Two major promoters are commonly used to express these fluorescence markers in mosquitoes—namely, the synthetic 3xP3 promoter (Berghammer et al. 1999) and the Ae. aegypti polyubiquitin promoter (PUb) (Anderson et al. 2010). The 3xP3 promoter appears to function quasiuniversally across insects (Horn et al. 2002) with the possible exception of tephritid flies (Schetelig and Handler 2013; Handler and Schetelig 2014). It drives expression primarily in the eyes, brain, and optic nerve of larvae and adults. In Aedes mosquito larvae, pupae, and adults, expression from this promoter can be weak (Fig. 1A–E). A fluorescence binocular microscope with excellent optics ensuring high sensitivity to the chosen fluorophore (CFP is often more difficult to visualize than GFP or YFP, although its mTurquoise2 derivative version [Goedhart et al. 2012] improves fluorescence intensity) and careful optical screening are essential to avoid missing weakly expressing transgenic individuals. The same promoter in Anopheles can yield stronger fluorescence than in Aedes (Fig. 1F,G), which, depending on the line and therefore on genomic position effects, will often extend to the entire ventral neural chain and anal papillae of the larvae more frequently than in Aedes. As a result, the 3xP3 promoter is well-suited for automated sorting of Anopheles larvae using a COPAS machine (Marois et al. 2012; Bernardini et al. 2014) but will generally be too weak for this purpose with Aedes larvae. In Aedes, the PUb promoter yields bright whole-body fluorescence (Fig. 1B–D,H) and makes it ideal for COPAS sorting.
Commonly used fluorescence markers for mosquito larvae. (A–D) Aedes albopictus pupae carrying combinations of three transgenic constructs, marked with 3xP3-mTurquoise (A), PUb-YFPvenus (B), and PUb-DsRed (C). (D) Shows the overlay of A–C. A negative pupa is indicated with dotted lines, and the arrowhead in A shows mTurquoise expression in the eye. (E) Fourth-instar Ae. albopictus larvae with 3xP3-mTurquoise expression in the eyes (arrowheads) and control larvae. (F) Fourth-instar Anopheles coluzzii larva with 3xP3-mTurquoise expression extending to the abdominal nerve chain and anal papillae. (G) Fourth-instar An. coluzzii larvae and pupae expressing 3xP3-EGFP and/or 3xP3-DsRed markers. The rightmost larva is oriented with the ventral side upward, showing GFP expression in the abdominal nerve chain, which is hidden from view in the other larvae. (H) Neonate Aedes larvae expressing PUb-GFP and wild types. Scale bars, 1 mm.
Unfortunately, the Aedes PUb promoter does not work well in Anopheles, except for weak expression in developing pupal wing veins, so that marker cassettes that incorporate the Aedes PUb promoter cannot be used in both of these mosquito genera. In Anopheles, an endogenous polyubiquitin promoter for ubiquitous expression has been described (Adolfi et al. 2018) and may be used as an alternative to 3xP3. The baculovirus OpIE2 promoter (Theilmann and Stewart 1992) can also offer a useful alternative, showing midgut and cardia expression in mosquito larvae, which becomes ubiquitous in late larvae, pupae, and adults. One drawback of OpIE2 is that, depending on its genomic insertion site, this promoter may become active only during late stages of larval development in some lines, which is problematic for COPAS sorting (optimal at the neonate stage, i.e., before larvae start feeding) as well as for optical sorting when screening neonate larvae is preferred. Several reporter constructs have been described in mosquitoes (e.g., Volohonsky et al. 2015), of which some could serve as alternative transgenesis selection markers, to avoid overlapping marker expressions in tissues of interest. Of note, the purpose of transgenesis can be to reveal the expression pattern of a newly investigated promoter by expressing a fluorescent reporter protein under its control. In this case, it is desirable to associate this reporter construct with a transgenesis marker showing no interference with the reporter (i.e., no overlapping fluorescence colors or fluorescence bleed-through). This can be achieved by choosing a transgenesis marker with weak or highly tissue-restricted expression, with very different fluorescence (e.g., associate a GFP or CFP reporter with a DsRed transgenesis marker, or associate YFP with CFP), or one that is nonfluorescent (puromycin resistance). Finally, if immunostaining experiments using various fluorophores are planned on tissue dissected from transgenic mosquitoes, it may be wise to choose a nonfluorescent transgenesis marker to avoid any interference with fluorescent staining. A puromycin resistance cassette (puromycin acetyltransferase gene cloned under control of the OpIE2 promoter) is efficient as a selection marker in Anopheles (Volohonsky et al. 2015). Unfortunately, it does not work for Aedes, whose larvae are naturally more resistant to puromycin. In this mosquito genus, a similarly convenient chemical selection marker remains to be developed.
ACKNOWLEDGMENTS
In our laboratory, research on mosquitoes is supported by funding from Inserm, CNRS, the University of Strasbourg, the city of Strasbourg, through contrat triennal “Strasbourg capitale européenne” 2018–2020 and Agence Nationale de la Recherche (ANR) through grants ANR-11-EQPX-0022, ANR-19-CE35-0007, and ANR-11-JSV3-0001.
Footnotes
-
From the Mosquitoes collection, edited by Laura B. Duvall and Benjamin J. Matthews.