Electroporation Loading and Flash Photolysis to Investigate Intra- and Intercellular Ca2+ Signaling
-
↵1 These two authors contributed equally.
Abstract
Many cellular functions are driven by variations in the intracellular Ca2+ concentration ([Ca2+]i), which may appear as a single-event transient [Ca2+]i elevation, repetitive [Ca2+]i increases known as Ca2+ oscillations, or [Ca2+]i increases propagating in the cytoplasm as Ca2+ waves. Additionally, [Ca2+]i changes can be communicated between cells as intercellular Ca2+ waves (ICWs). ICWs are mediated by two possible mechanisms acting in parallel: one involving gap junctions that form channels directly linking the cytoplasm of adjacent cells and one involving a paracrine messenger, in most cases ATP, that is released into the extracellular space, leading to [Ca2+]i changes in neighboring cells. The intracellular messenger inositol 1,4,5-trisphosphate (IP3) that triggers Ca2+ release from Ca2+ stores is crucial in these two ICW propagation scenarios, and is also a potent trigger to initiate ICWs. Loading inactive, “caged” IP3 into cells followed by photolytic “uncaging” with UV light, thereby liberating IP3, is a well-established method to trigger [Ca2+]i changes in single cells that is also effective in initiating ICWs. We here describe a method to load cells with caged IP3 by local electroporation of monolayer cell cultures and to apply flash photolysis to increase intracellular IP3 and induce [Ca2+]i changes, or initiate ICWs. Moreover, the electroporation method allows loading of membrane-impermeable agents that interfere with IP3 and Ca2+ signaling.
FROM INTRA- TO INTERCELLULAR Ca2+ SIGNALING
The importance of Ca2+ as a second messenger in the cell is emphasized by the observation that even the most primitive prokaryotes express a variety of active Ca2+ pumps and passive channels or transporters that, respectively, create and dissipate the Ca2+ gradient across the plasma membrane. The multiplicity of messengers and channels existing today brings up a picture of a highly complex Ca2+ signaling toolkit tuned to fulfill individual cellular needs throughout vertebrate life, from fertilization through development to death and including the processes of cell proliferation, differentiation, neurotransmitter release, secretion, synaptic plasticity, gene expression, immune responses, muscle contraction, cardiomyocyte function, endothelial permeability, apoptosis, and many others (Berridge 1997; Case et al. 2007; Iino 2010).
All eukaryotic cells control their intracellular, cytosolic Ca2+ concentration ([Ca2+]i) through an intimate interplay between (i) Ca2+ entry from the extracellular space, (ii) Ca2+ release from intracellular storage sites, (iii) Ca2+ sequestration into the endoplasmic reticulum (ER) and other organelles, and (iv) Ca2+ extrusion out of the cells. The free [Ca2+]i in resting cells varies between 50 nm and 100 nm (Hess et al. 1989; Koenig et al. 1989) and represents only a small part of the total cellular Ca2+, which largely resides in the ER, where ∼70% of the cellular Ca2+ can be found (Wood and Gillespie 1998). Several Ca2+-permeable channels have been put forward as ER Ca2+ release routes, with inositol 1,4,5-trisphosphate receptors (IP3Rs) and ryanodine receptors (RyRs) as major contributors. Binding of the second messenger IP3 to the IP3R increases its open probability, resulting in Ca2+ release from the ER, down its electrochemical gradient (Streb et al. 1983; Burgess et al. 1984; Berridge et al. 2003). Several isoforms of phospholipase C (PLCβ, PLCγ, PLCδ, PLCϵ, PLCζ), which are activated by different mechanisms, catalyze the cleavage of phosphatidylinositol bisphosphate (PIP2) into IP3 and diacylglycerol (DAG) (Rhee 2001; Yoon et al. 2008).
Ca2+ signals are highly organized in time and in space; at the temporal level, [Ca2+]i changes may be oscillatory, appearing as repetitive Ca2+ spikes that result from the periodic cycling of Ca2+ between the cytoplasm and the internal Ca2+ stores. A bimodal regulation of the open probability of the IP3R by [Ca2+]i lies at the basis of Ca2+ oscillations (Bezprozvanny et al. 1991; Zhang and Muallem 1992; Patel et al. 1999; Iino 2000). More specifically, low [Ca2+]i exerts a positive feedback on IP3R Ca2+ release whereas a negative feedback is provided by high (>200 nm) [Ca2+]i (Bezprozvanny et al. 1991). Spatial organization, on the other hand, refers to the localized nature of these signals as elementary events termed Ca2+ blips or puffs. The fusion of these events results in an intracellular, regenerative Ca2+ wave, eventually merging into a global [Ca2+]i increase (Berridge 2006; Dupont et al. 2007). Moreover, intracellular waves are not only confined to the cytoplasm of one cell but can propagate to neighboring cells as an intercellular Ca2+ wave (ICW) (Leybaert and Sanderson 2012). This intercellular process allows for the transmission of local Ca2+ signals to a global multicellular level, thereby synchronizing the function of a large group of cells.
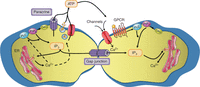
Two mechanisms support the intercellular propagation of Ca2+ signals (Fig. 1). The first one involves a direct cell-to-cell communication pathway between neighboring cells established by vast arrays of plasma membrane channels called “gap junctions” (GJs). GJ channels are composed of two hemichannels, each consisting of six transmembrane connexin (Cx) proteins. More than 20 Cx species have been cloned from rodents and humans. Their nomenclature is based on their molecular mass, which ranges from 25 to 62 kDa. Of note, hemichannels may also be formed by pannexins (Panxs), but these are less likely to assemble into GJs (Penuela et al. 2007; Sosinsky et al. 2011). GJs allow the passive diffusion of small (<1–1.5 kDa) hydrophilic molecules including IP3 or Ca2+ itself between the cytoplasm of neighboring cells (Boitano et al. 1992; Saez et al. 2003; Dupont et al. 2007; D’Hondt et al. 2009; Leybaert and Sanderson 2012). IP3 is a soluble, low-molecular-mass molecule (486 Da) that easily diffuses in the cytoplasm and can pass through GJ channels. Ca2+ can also be transferred via GJs but, in contrast to IP3, Ca2+ diffusion in the cytoplasm is limited because of its extensive buffering by cytoplasmic Ca2+-binding proteins that diffuse more slowly (Allbritton et al. 1992). The diffusion constant of Ca2+ is ∼15 times smaller than that of IP3 (∼20 µm2/sec vs. ∼300 µm2/sec), and the GJ permeability of IP3 is 100 times larger than that of Ca2+(Allbritton et al. 1992; Hofer et al. 2002). Furthermore, several studies have indicated that increasing Ca2+ in single cells, including hepatocytes, astrocytes, airway epithelial cells, and cochlear cells, does not trigger substantial ICW activity (Leybaert et al. 1998; Clair et al. 2001; Beltramello et al. 2005). As a consequence, IP3 is considered the primary intercellular messenger (Boitano et al. 1992). The second mechanism for ICW propagation is based on paracrine signaling and involves the release of a Ca2+ mobilizing messenger, in most cases ATP, which diffuses into the extracellular space, binds to its receptors on neighboring cells, and activates downstream signaling cascades that lead to a [Ca2+]i increase in the target cell (Hassinger et al. 1996). Release of Ca2+ mobilizing messengers has been documented to occur via multiple pathways including exocytosis, diffusion through either Panx or Cx hemichannels (hemi-GJs positioned as pores that link the cytoplasm to the extracellular environment), or P2X7 receptor channels (Stout et al. 2002; Braet et al. 2003; Bezzi et al. 2004; Gomes et al. 2005; Locovei et al. 2006; Montana et al. 2006). The downstream effect of these messengers involves the generation of IP3 through the activation of G protein-coupled receptors (GPCRs), or Ca2+ entry via plasma membrane channels (Cornell-Bell et al. 1990; Scemes et al. 2000). Most frequently, wave propagation is sustained by both the gap junctional and paracrine pathways (Paemeleire et al. 2000; Scemes et al. 2000; Braet et al. 2001), and is furthermore supported by regenerative steps, which result in more extensive wave propagation. ICWs that rely on a passively installed gradient of intracellular IP3 or of extracellular ATP (or alternative messengers) are expected to have limited propagation due to messenger degradation and desensitization effects. Signal regeneration may occur as a consequence of the [Ca2+]i increase that potentiates the synthesis of IP3 in the cell or activates the release of ATP into the extracellular space. Regeneration of IP3 can be brought about by Ca2+-activation of PLCδ (Hofer et al. 2002). Several ATP release routes such as vesicular release and diffusive release via Cx and Panx hemichannels are also dependent on [Ca2+]i (Locovei et al. 2006; Montana et al. 2006; De Vuyst et al. 2009). In general, ICWs rely on a combination of passive and regenerative mechanisms (Leybaert and Sanderson 2012).
Mechanisms underlying the propagation of intercellular Ca2+ waves. Ca2+ waves can be propagated by the intercellular transfer of signaling molecules, such as IP3, through gap junctions. The extracellular pathway involves the release of different molecules, primarily ATP, via hemichannels, a vesicular pathway, or channels such as the P2X7 receptor. ATP diffuses to neighboring cells where it activates GPCRs or Ca2+-entry channels, thereby increasing [Ca2+]i. Regenerative mechanisms (activation of PLC or stimulation of ATP release mechanisms) are indicated by the dashed arrows.
ICWs have been extensively characterized in adherent cell monolayer cultures of various cell types (e.g., glial cells, endothelia, hepatocytes) where they generally spread with a velocity of 10–20 µm/sec (Dupont et al. 2007; Iino 2010; Leybaert and Sanderson 2012). Over the last decade, ICWs have also been shown under ex vivo conditions based on the work performed in brain slices, isolated blood vessels, the perfused liver, and in vivo in the brain (Robb-Gaspers and Thomas 1995; Schipke et al. 2002; Hirase et al. 2004; Parthasarathi et al. 2006; Hoogland et al. 2009). Numerous functions have been proposed for ICWs, and studies performed in the retina, the inner ear, the brain, the liver, and blood vessels have brought up some supportive evidence (Leybaert and Sanderson 2012). These include a role of glial cell ICWs in the proliferation and differentiation of neural progenitor cells in the developing retina (Pearson et al. 2005), and of inner ear supporting cell ICWs in the control of hearing sensitivity (Beltramello et al. 2005; Anselmi et al. 2008). In the brain, astrocytes display ICWs but neurons and vascular cells may also be involved (Hirase et al. 2004; Haydon and Carmignoto 2006; Scemes and Giaume 2006). Astrocytic extensions are in close contact with neuronal synapses (together forming tripartite synapses) and with brain microvessels via endfeet. At both astrocytic endings, [Ca2+]i changes have profound effects on synaptic and vascular functioning respectively, triggering gliotransmitter release (Ben Achour et al. 2010; Stehberg et al. 2012) at the synapse side and the release of vasoactive substances at the vessel side (Attwell et al. 2010). At the blood-brain barrier (BBB), astrocytic [Ca2+]i changes may influence endothelial cells and thus BBB function (Braet et al. 2001; Leybaert 2005). ICWs are expected to activate these Ca2+-dependent processes, but it is actually not known whether this can occur in a physiological context in vivo. ICWs can be initiated in the in vivo retina and brain by various triggers; however, an important concern is that such experimental stimulation (electrical, specific receptor ligands, photoactivation of Ca2+—see below) is often much stronger than can be reasonably expected under normal physiological conditions. Spontaneous ICWs have been reported in the cerebellum in vivo (Hoogland et al. 2009), but the wave activity seems to disappear when the animals start locomotor activity (Nimmerjahn et al. 2009).
Pathological conditions are likely to provide stronger cellular and molecular stimuli for ICWs compared to the physiological situation. We recently found that BBB endothelial cells exposed to low extracellular Ca2+ conditions, which can occur in epilepsy, stroke, and brain trauma, display continuous ICW activity that is associated with increased permeability of the BBB (De Bock et al. 2012a). We concluded from this work that endothelial ICWs disrupt the BBB more profoundly than endothelial Ca2+ oscillations occurring in approximately the same cell mass (De Bock et al. 2011). Cell death is another example where ICWs may play a role: IP3 and [Ca2+]i changes are intimately linked to the apoptotic process (Decrock et al. 2009, 2012; Monaco et al. 2012) and we recently reported that IP3 diffusion via Cx43 GJs is crucial to provoke apoptosis in adjacent cells (Decrock et al. 2012). Thus, ICWs may be implicated in the communication of cell death to surrounding healthy cells. Inflammation provides another rather unexplored context where ICWs may be implicated: ICWs propagated via Cx43 channels trigger a proinflammatory phenotype in endothelial cells from lung blood vessels in response to local injury (Parthasarathi et al. 2006). Finally, in the brain, ICWs have been reported in astrocytes upon exposure to focal ischemia conditions (Ding et al. 2009) and in the vicinity of amyloid β plaques in Alzheimer disease mouse models (Kuchibhotla et al. 2009). Further research is needed to explore the mechanisms underlying the increased appearance of ICWs in these and other pathological conditions and to determine their contribution in the pathogenesis and disease progression.
METHODS TO INDUCE INTERCELLULAR Ca2+ WAVES
Various stimuli have been used to generate ICWs and these can be divided into focal stimuli (locally applied to a single/group of cell(s)) or global stimuli (applied over a whole cell culture or tissue) (Leybaert and Sanderson 2012). Local stimulation necessitates a spatially restricted application of chemical compounds such as ATP or bradykinin that increase IP3 and/or [Ca2+]i via a plasma membrane receptor signaling pathway. Global stimulation can also be triggered by chemical agonists or conditions, for example by applying glutamate to glial cells (Cornell-Bell et al. 1990), serotonin to blow fly salivary glands (Zimmermann and Walz 1997), or by reducing the extracellular Ca2+ concentration (Zanotti and Charles 1997; Domenighetti et al. 1998; Isshiki et al. 1998). Local mechanical stimulation of a single cell with a micropipette or piezo-electric device has been frequently used in the past (Charles et al. 1991; Boitano et al. 1992; Paemeleire et al. 2000; Braet et al. 2001); the resulting deformation of the plasma membrane triggers the production of IP3. Major disadvantages of the technique are (i) the poor reproducibility of the stimulus intensity, (ii) the possible induction of plasma membrane rupture with consequent release of intracellular constituents, and (iii) the activation of various other signal transduction pathways that may influence ICW properties (Paemeleire et al. 2000). A better alternative for local stimulation is the introduction of specific messengers of the Ca2+-signaling pathway, for example microinjection of IP3 (Niessen et al. 2000; Beltramello et al. 2005) or the use of a biologically inactive precursor of IP3 or Ca2+ (respectively, caged IP3 or caged Ca2+) by microinjection, scrape loading, AM-ester loading or in situ electroporation (Giovannardi et al. 1998; Braet et al. 2001, 2004; Leybaert and Sanderson 2001). The ICW is then initiated by exposure of the caged compound to UV light, which uncages and liberates the active compound. This method provides an efficient and controllable way, both at the spatial and temporal level, to trigger an increase of cytosolic IP3 with subsequent Ca2+ release from the stores. Three categories of caged compounds can be distinguished: Ca2+ chelators (e.g., caged EGTA or EDTA), Ca2+ ionophores (e.g., caged A23187), and second messengers (e.g., caged IP3 or cAMP). Of note, the use of microinjection of IP3, as well as localized photoactivation of inactive IP3 precursors by flash photolysis, has provided strong evidence for the role of IP3 in ICW propagation between cells (Boitano et al. 1992; Leybaert et al. 1998; Niessen et al. 2000; Leybaert and Sanderson 2001; Beltramello et al. 2005).
ELECTROPORATION LOADING AND FLASH PHOTOLYSIS OF CAGED IP3 FOR STUDYING INTRA- AND INTERCELLULAR Ca2+ SIGNALING
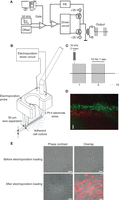
Electroporation loading of caged IP3 or caged Ca2+ with subsequent flash photolysis has been extensively applied to study the properties of ICWs (Leybaert and Sanderson 2001; Braet et al. 2004; Decrock et al. 2012). Electroporation loading uses short high-voltage pulses to temporarily increase the permeability of the plasma membrane, thereby giving access of extracellular molecules to the cells’ interior. It is hypothesized that application of the electrical field transiently creates micropores (50–120 nm) in the plasma membrane (Teruel and Meyer 1997; Gehl 2003). Importantly, in situ electroporation avoids a permanent impalement of the plasma membrane, in contrast to microinjection. Originally developed for gene transfer, electroporation is now used to load cells with a variety of substances including ions, drugs, dyes, radiotracers, antibodies, oligonucleotides, and RNA/DNA (Ho and Mittal 1996; Gehl 2003). The very first electroporation procedures were focused on bacterial DNA transfer and largely ignored cell viability; however, further optimization has led to electroporation protocols that combine high cell viability with high loading efficiency (Teruel and Meyer 1997; Gehl 2003). Efficient electropermeabilization depends on different variables of which the electric pulse parameters are the most important. The most optimal electrical pulse protocol currently available is a high-frequency (50 kHz) oscillating electrical signal that is not associated with a net direct current (DC) component, that is, a voltage oscillating around the zero potential as a bipolar alternating current (AC). The choice of the particular 50 kHz frequency is based on the work of Tekle et al. (1991) and follow-up work from Boitano et al. (1992). Compared to unipolar electroporation, bipolar pulsed electroporation combines good loading efficiency with minimal impact on cell viability (Tekle et al. 1991; Kotnik et al. 2001; De Vuyst et al. 2008). We developed a device to deliver 50 kHz AC-coupled stimulation (Fig. 2A) and combined this with an electrode that allows in situ electroporation loading of a defined zone of cells within an adherent cell culture (Fig. 2B). AC-coupling is practically achieved by isolating the output of the signal-generating device by means of a transformer (Fig. 2A). It is important to stress here that the DC component in the electrical drive signal to the electrode is a critical determinant in cell death following electroporation; any residual presence of such DC component may result in increased cell death scores. The 5 kHz voltage oscillations are applied in a pulsed and repeated manner (Fig. 2C): the oscillatory signal is switched-on ten times per second for 2 msec, forming a pulse train; the pulse train is repeated 15 times with 0.5 sec pauses. The area of electroporated cells depends on the electrode dimensions; we currently use two parallel Pt–Ir wires 8 mm long separated by 500 µm. Placing the electrode ∼400 µm above the cells (Fig. 2B) and applying a peak-to-peak oscillation voltage of 100 V results in an electroporated zone of ∼300 µm wide and 8 mm long (Fig. 2D). The actual size of the electrode can be modified according to experimental needs. Single-cell electroporation is in principle also possible when using a locally positioned micropipette as one electrode and a bath Ag/AgCl pellet as the other electrode. Using this approach, cells can be loaded with a variety of membrane-impermeable molecules, including fluorescent dyes, caged compounds, molecules/peptides interfering with signaling pathways, and even recombinantly expressed and purified enzymes (Braet et al. 2001, 2003, 2004; De Vuyst et al. 2008; Decrock et al. 2009, 2012; De Bock et al. 2012b; Monaco et al. 2012). Importantly, enzyme activity remains intact when introducing them into cells, as emphasized by the ability of a human type I IP3 5-phosphatase to reduce IP3-induced Ca2+ increases after its electroporation loading into C6 glioma cells (Decrock et al. 2012). Results show that the loading efficiency increases with the applied voltage, which is most often expressed as the electrical field strength. A threshold of ∼800 V/cm (i.e., 40 V peak-to-peak voltage applied over electrode wires that are 500 µm apart) is necessary to obtain a detectable degree of electroporation. With a field strength of 2000–2800 V/cm, a loading efficiency on the order of 20%–30% can be realistically obtained for molecules with a molecular mass in the range of 0.4–10 kDa. The loading efficiency decreases with molecular mass and is in the order of 10% for substances with a molecular mass of 40 kDa. Importantly, cell viability is well preserved and cell death in the electroporation zone is low and not different from nonelectroporated zones (Table 1, Fig. 2E) (De Vuyst et al. 2008; Decrock et al. 2009). The use of this electroporation technique is described in the associated protocol, Electroporation Loading of Membrane-Impermeable Molecules to Investigate Intra- and Intercellular Ca2+ Signaling (Decrock et al. 2015a).
Electroporation setup to load an adherent cell culture. (A) Schematic overview of the generator and amplifier setup used for bipolar electroporation (FB signifies feedback circuit). Detailed diagrams and amplifier/driver box are available on request. (B) Diagram illustrating the electroporation electrode consisting of two Pt–Ir wires (Ø 120 µm, 500 µm separation). During the experimental procedure, the electrode is positioned above the cells. (C) Bipolar pulse protocol. The electroporation driver circuit produces a 50-kHz oscillating signal alternating between positive and negative polarity. This oscillating signal is applied in 15 series of 1 sec duration, each consisting of 10 repetitions of 2 msec duration. (D) Illustration of dual electroporation loading of C6 glioma cells stably transfected with Cx43. Cells were first electroporation-loaded with Dextran Texas Red 10 kDa (DTR, red color), followed by loading of the adjacent area with FITC-dextran 10 kDa (green color). Scale bar, 200 µm. (E) Phase contrast/fluorescence overlay images taken before and directly after electroporation loading demonstrating that cell morphology and viability is well preserved in the electroporation zone. Scale bar, 50 µm.
Effect of electroporation of C6 glioma cells with a field strength of 2000 V/cm on cell death quantified by various markers
Over the past years, we have exploited the unique characteristics of this electroporation technique in intra- and intercellular Ca2+ signaling research by loading small localized areas within monolayer cell cultures with several membrane- and GJ-impermeable inhibitors of the Ca2+ signaling toolkit (Table 2). More specifically, we loaded caged IP3 together with the IP3-degrading enzyme type I IP3 5-phosphatase to determine the efficiency with which this enzyme metabolizes IP3 (Decrock et al. 2012). Similarly, we loaded the antiapoptotic BH4 domain of the B-cell lymphoma 2 (Bcl-2) as an IP3R inhibitor in C6 glioma cells. Both substances successfully suppressed [Ca2+]i changes brought about by the photolytic release of IP3 from its caged precursor (Decrock et al. 2012; Monaco et al. 2012). Cytochrome C (CytC) has been shown to alleviate the negative feedback of Ca2+ on IP3Rs by binding to the carboxy-terminal tail of IP3R type 1 (amino acids 2621–2636) (Boehning et al. 2003). Consequently, we have used loading of CytC into Madin Darby Canine Kidney (MDCK) cells to show that abolishing the negative feedback of Ca2+ on the IP3R inhibits Ca2+ oscillations triggered by extracellulary applied ATP or bradykinin (De Bock et al. 2012b). Importantly, the low concentrations of CytC did not induce any detectable apoptosis during the 15 min time period of these experiments. Moreover, a peptide corresponding to the CytC-binding residues of the IP3R type 1 (IP3RCYT peptide) abolished the CytC–IP3R interaction and the inhibitory effect of CytC on the Ca2+ oscillations when coloaded in this region (Boehning et al. 2005; De Bock et al. 2012b). Of note, in situ electroporation loading of a defined zone within a cell culture with Ca2+-interfering agents gives the advantage that the responses in loaded cells can be compared to those observed in nonloaded cells, giving a more “paired” experimental approach.
Examples of applications of electroporation-loading of membrane-impermeable inhibitors of the Ca2+ signaling toolkit as used in intra- and intercellular Ca2+ signaling research
We have applied electroporation loading of nonpermeable peptides to show that Cx hemichannels play a role in bradykinin-triggered Ca2+ oscillations. The opening of Cx43 hemichannels is dependent on [Ca2+]i in a bimodal manner with [Ca2+]i < 500 nm potentiating hemichannel opening and [Ca2+]i > 500 nm inhibiting it (De Vuyst et al. 2006, 2009). This bimodal (bell shaped) Ca2+ dependence resembles the Ca2+ dependence of IP3R channel open probability (Bezprozvanny et al. 1991). Thus, we hypothesized that Ca2+ entry via Cx43 hemichannels is promoted by moderate [Ca2+]i elevation and inhibited by stronger [Ca2+]i increases, thereby contributing as an additional Ca2+ oscillation mechanism acting in parallel with the IP3R. The negative Ca2+ feedback on Cx43 hemichannel opening can be alleviated by a short peptide mimicking the last nine amino acids of Cx43 (Ponsaerts et al. 2010). We found that electroporation loading of the cells with this peptide suppressed bradykinin-induced Ca2+ oscillations in MDCK cells (De Bock et al. 2012b).
The list of substances interfering with Ca2+ signaling that can be safely loaded into cells by electroporation is not limited to these few examples and also includes agents with several tens of kiloDalton molecular mass. Plasma membrane-impermeable Ca2+ buffers such as BAPTA-dextran (molecular mass 70 kDa) can be loaded (Leybaert et al. 1998). In addition, electroporation loading of certain IP3-absorbent proteins which are based on the (modified) IP3-binding domain of the mouse or human IP3R type 1 (amino acids 224–604/5, respectively) (Uchiyama et al. 2002; Lin et al. 2005) or on the pleckstrin homology domain (amino acids 95–233) of the PLC-like 130 kDa protein p130 isolated from rat brain (Varnai et al. 2002; Lin et al. 2005), would be a valuable extension of the toolset for studying IP3-dependent Ca2+ signaling. Saheki et al. used a single-cell electroporation technique to load plasmid DNA encoding an IP3 absorbent into astrocytes. In this way, they were able to inhibit IP3-induced Ca2+ release specifically in astrocytes in an astrocyte–neuron coculture system (Saheki et al. 2005).
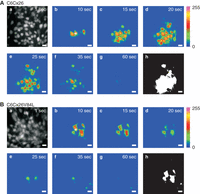
The previous examples concerned work that aimed to interfere with intracellular Ca2+ signaling. Electroporation loading of caged IP3 and flash photolysis has been elegantly used in the past to trigger ICWs (Leybaert et al. 1998), also see Flash Photolysis of Caged IP3 to Trigger Intercellular Ca2+ Waves (Decrock et al. 2015b). Using a similar approach we have recently showed that ICWs initiated by flash photolysis of caged IP3 are strongly inhibited in C6 glioma cells expressing mutant Cx26V84L as compared to wild-type Cx26 (Fig. 3) (Decrock et al. 2012). GJs formed by this mutant Cx26 (which is found in certain cases of nonsyndromic hearing loss) have been shown to display impaired permeability to IP3 (Beltramello et al. 2005). Importantly, the valine residue is conserved in most α- and β-group Cxs, making this an attractive approach to reduce the IP3 permeability of GJs composed of other Cxs.
Intercellular Ca2+ wave propagation induced by photolysis of electroporation-loaded caged IP3 in C6 glioma cells expressing wild type Cx26 and V84L mutant Cx26 that has impaired IP3 permeability. (A) Images displaying Ca2+ wave propagation in C6 cells transduced with wild-type Cx26. Image a is a gray scale image of the cells after loading with the Ca2+ indicator Fluo-3. Caged IP3 was photolytically released 20 msec before recording image b. Images b–g were taken at time points as indicated and were subsequently subtracted by image a to generate difference images shown in pseudocolor. Image h is a calculated image displaying the reacting cells (white cells) that showed a Fluo-3 fluorescence change >5% above the baseline signal. The UV beam was focused to the spot marked with a star in image b. The area of cells contributing to the Ca2+ wave can be estimated from the surface area of white cells. (B) Similar experiment as in A, but now performed on C6 cells transduced with V84L mutant Cx26 that are characterized by an impaired gap junctional permeability to IP3. The area of reacting cells was strongly reduced. The experiments were performed in the presence of the ATP-degrading enzyme apyrase in the extracellular solution, to suppress the extracellular pathway of Ca2+ wave communication. Scale bar, 20 µm.
In situ electroporation loading can also be used to load two different cell zones within a single-cell culture dish (Fig. 2D). In this way, a first zone can be loaded with caged IP3 (“trigger zone”), while a second zone may be loaded with substances interfering with the Ca2+ machinery (“recipient zone”). Thus, responses to the photolytic release of IP3 in the trigger zone can be investigated in the recipient area. Both the Cx26V84L expressing cells and the dual electroporation protocol were of utmost importance in a recent study where we showed that the transfer of IP3 through GJs is crucial for the communication of cell death from cells undergoing apoptosis to neighboring healthy cells (Decrock et al. 2012).
ACKNOWLEDGMENTS
This work is supported by the Fund for Scientific Research Flanders (FWO-Vlaanderen), Belgium (grant nos. G.0140.08, G.0134.09, G.0298.11 and G.0571.12) and the Interuniversity Attraction Poles Program (Belgian Science Policy, projects P6/31 and P7). E.D. is a postdoctoral research fellow of the Fund for Scientific Research Flanders (FWO-Vlaanderen), Belgium.
- © 2015 Cold Spring Harbor Laboratory Press