Mosquito Transposon-Mediated Transgenesis
- 1Department of Microbiology and Molecular Genetics, University of California, Irvine, California 92697-4500, USA
- 2Department of Molecular Biology and Biochemistry, University of California, Irvine, California 92697-3900, USA
- ↵3Correspondence: aajames{at}uci.edu
Abstract
Transposon-mediated transgenesis of mosquito vectors of disease pathogens followed the early success of transgenesis in the vinegar fly, Drosophila melanogaster. The P transposable element used in Drosophila does not function canonically in mosquitoes, and repeatable, routine transgenesis in mosquitoes was not accomplished until new transposable elements were discovered and validated. A number of distinct transposons were subsequently identified that mediate the introduction of exogenous DNA in a stable and heritable manner in mosquito species, including members of the genera Aedes, Anopheles, and Culex. The most versatile element, piggyBac, is functional in all of these mosquito genera, as well as in many other insects in diverse orders, and has been used extensively outside the class. Transposon-mediated transgenesis of recessive and dominant marker genes and reporter systems has been used to define functional fragments of gene control sequences, introduce exogenous DNA encoding products beneficial to medical interests, and act as “enhancer traps” to identify endogenous genes with specific expression characteristics.
BACKGROUND
Following the discovery that the P element could be used to efficiently transform Drosophila melanogaster (Rubin and Spradling 1982), concerted efforts were made to develop transposon-based transgenic technologies for a wide variety of arthropod species of agricultural and public health significance. Early efforts in mosquitoes focused unproductively on trying to adapt the P element to several species (Miller et al. 1987: McGrane et al. 1988; Morris et al. 1989), but it was not until the discovery of a number of new elements that success was finally achieved. “Plasmid mobility assays” were used to determine whether or not a particular element could be mobilized (excise and insert) in the germline of a specific insect (O'Brochta and Handler 1988). These assays clearly showed that despite its power in D. melanogaster, the P element could not transform the vast majority of other, non-drosophilid species in which it was tested. Efforts were made to use the P element by identifying host factors necessary for activity in D. melanogaster (Rio and Rubin 1988; Handler et al. 1993), but the parallel discovery and testing of new elements ultimately led to their adoption.
Work in mosquitoes has focused on Class II transposable elements, which mobilize through a DNA intermediate and can result in either conservative (no increase in element copy number) or replicative (results in an increase in copy number of the element) transposition (Atkinson and James 2002). Class II elements have variable-length terminal repeat DNA sequences, which are most often inverted (designated inverted terminal repeats [ITRs] or terminal inverted repeats [TIRs]). These repeats flank an open reading frame encoding a transposase that recognizes the ITRs and a conserved target site 2–8 bp in length and catalyzes the mobility of the element from one site in a plasmid or chromosome to another (Fig. 1). Following successful testing in mobility assays, the elements Mos1 mariner, Minos, and Hermes (Franz and Savakis 1991; Medhora et al. 1991; Warren et al. 1994) were adapted to transform mosquito species (Table 1; Sarkar et al. 1997; Coates et al. 1998; Jasinskiene et al. 1998; Catteruccia et al. 2000a,b; Allen et al. 2001). Molecular characterization of the baculovirus insertion element IFP2 using mobility assays in insect cells set the groundwork for the element now known as piggyBac to be applied widely across mosquito species (Fraser et al. 1995; Elick et al. 1997). Currently, piggyBac is sufficiently versatile to be useful across species from yeast to mammals (Yusa 2015) and is now the element used predominantly for transposon-mediated transgenesis of all the major mosquito vectors (Table 1).
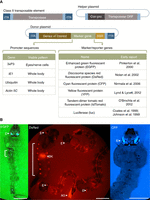
General organization and use of Class II transposable elements. (A) Schematic representations of a Class II transposable element and engineered helper and donor plasmids. Active Class II transposable elements have inverted terminal repeated (ITR) DNA sequences flanking a functional transposase gene complete with promoter and nontranscribed control DNA (Transposase). The helper plasmid (Helper) has the transposase open reading frame (ORF) under the control of a constitutive promoter (Con-pro). The donor plasmid (Donor) contains ITRs flanking genes of interest, a dominant marker gene along with control sequences to allow visible screening of transgenic animals, and in some cases sites for site-specific recombination (SSR). Both donor and helper DNA sequences are cloned into bacterial plasmids (thin lines) that allow production and purification for use in embryo microinjections or other DNA-introduction technologies. (Table) Examples of promoter sequences and marker/reporter genes along with early- or first-use citations in mosquitoes. (B) Examples of transposon-mediated transgenic mixed-instar Anopheles stephensi mosquito larvae marked with 3xP3-fluorescence genes. All larvae show transgene-mediated fluorescence in their eyes (E). Some larvae exhibit additional transgene-specific fluorescence in the segmented nervous tissue (vertical brackets), and anal gills (AG). The food bolus in the gut can produce background (BK) fluorescence. Horizontal bars, ∼1 mm. (EGFP) Enhanced green fluorescent protein, (CFP) cyan fluorescent protein, (DsRed) Discosoma species red fluorescent protein.
Transposable elements (A) and common promoters (B) used in mosquito transgenesis
Transposon-mediated mosquito transgenesis experiments have focused primarily on defining functional fragments of gene control sequences (promoters, 5′- and 3′-end DNAs, introns), introducing exogenous DNA encoding products beneficial to mitigating pathogen transmission, modifying expression profiles of endogenously derived genes, and introducing “enhancer traps” to identify endogenous genes with specific expression characteristics. Enhancer traps based on piggyBac were used to identify genes with potentially useful expression profiles (O'Brochta et al. 2012; Reid et al. 2018). Functional promoter analysis studies were biased at the outset by looking at genes that were expressed in mosquito tissues important for their role as pathogen vectors. This bias was expected to reduce potential transgenesis-related fitness costs by restricting the expression of transgenes to the infection-relevant sex, developmental stage, and mosquito body compartments in which the pathogens are found. To date, transgenesis technologies have identified gene sequences that allow stage-, tissue-, and sex-specific expression of dominant marker and reporter genes, and DNA sequences whose products interfere with viral and protozoan pathogen development and transmission (Table 1). Future studies could identify effective and robust regulatory sequences that are uniquely activated in response to pathogen infection in order to minimize even further any fitness issues associated with transgenesis. Additionally, reporter strains for binary expression systems (e.g., QF2) can be generated easily through transposon-mediated transgenesis and are useful for the characterization of neural circuits and neuronal activity visualization (Lynd and Lycett 2012; Riabinina et al. 2016).
EXPERIMENTAL DESIGN CONSIDERATIONS
Element choice is significant for transposon-based experiments. Although all elements have a preferred target nucleotide sequence for integration, these vary in size and complexity (Table 1). Both Minos and Mos1 recognize the dinucleotide TA, which occurs frequently enough in the genome to make integrations of these elements appear random. piggyBac and Hermes recognize longer target sequences, four (TTAA) and eight bases (GTNCAGAC) in length, respectively, but these sequences also occur frequently enough in the genome to appear to be distributed randomly. In our experience with Aedes aegypti, Mos1 tends to insert in fewer copy numbers in the genome than piggyBac, making post-transformation characterization of the transgenes easier to manage (V Bottino-Rojas and AA James, unpubl.). However, Anopheles transgenesis is almost exclusively performed with piggyBac. Elements can exhibit noncanonical and unstable integration and postintegration behavior, making it important to accurately characterize the insertion copy number, structure, and stability in individual transgenic lines (Jasinskiene et al. 2000; Adelman et al. 2004; Palavesam et al. 2013).
Transposon-mediated transgenesis is usually performed with a binary system of donor and helper plasmids (Fig. 1). The donor plasmid contains ITRs flanking the transgene, whereas the helper plasmid transiently expresses the transposase enzyme, which catalyzes element excision from the donor plasmid and insertion into the host genome (Fraser et al. 1996). Our associated protocol provides detailed experimental considerations (see Protocol: Generating and Validating Transgenic Mosquitoes with Transposon-Mediated Transgenesis [Bottino-Rojas and James 2023]). Plasmid cloning allows the production and purification of large amounts of DNA before experimental usage. The minimal donor plasmid design contains the ITRs flanking a marker gene and genes of interest. Target sequences for site-specific recombination (SSR) also can be included that allow manipulation of the transgene insert once it is integrated into the genome. DNA-insulating sequences can be added just inside the ITRs to help mitigate insertion-site effects (Carballar-Lejarazú et al. 2013). Fully functional mosquito-derived introns have been used to modify gene-of-interest configurations when needed (Franz et al. 2006). Other components of the genetic toolbox (e.g., Gal4-UAS systems, SSR docking sites) are discussed elsewhere (see Topic Introduction: Genetic Toolbox Approaches in Mosquitoes [Riabinina et al. 2022]). The exact order of the genes in the element depends on the purpose of the experiment. The success rate of transposase-mediated mobilization from a plasmid into a mosquito genome is likely to depend on the size of the DNA being inserted, but a meta-analysis of the published work showed that most elements can efficiently insert 10–15 kb of DNA (Gregory et al. 2016).
Helper plasmids contain the transposase open reading frame (ORF) under the control of a constitutive promoter cloned into a plasmid. Heat-shock protein gene promoters (hsp70 from D. melanogaster and hsp82 from Drosophila pseudoobscura; Table 1) are active constitutively in mosquito embryos and have been used successfully in many transgenesis experiments. Helper plasmids will not replicate in the insect embryo and are expected to be lost by dilution as the injected animals progress through development.
Purified transposases also have been used in place of a helper plasmid (Coates et al. 2000). Hyperactive transposase proteins were shown to improve transformation rates in nonmosquito species, and transposase mRNAs can be injected when helper plasmids are not readily available (Otte et al. 2018).
A key contribution to mosquito transgenic technology was the discovery and application of dominant-acting fluorescent proteins that can be assayed in live animals and are visible when expressed from a single copy of their encoding gene (Fig. 1; Handler and Harrell 2001). Genes encoding proteins that fluoresce in green, red, and blue wavelengths were adapted quickly as marker genes in mosquitoes and have been used extensively. These gene products also can be used as reporter sequences in both qualitative and quantitative assays. Indeed, characterization of specific gene control DNA (promoters, 5′- and 3′-end untranslated regions [UTRs]) has been performed successfully for many genes by linking the predicted control elements to one of the fluorescent protein–encoding reading frames and scoring activity in live animals under fluorescence microscopy (Table 1).
DISCUSSION
Transposon-mediated generation of genetically engineered mosquito vectors of disease has revolutionized both basic and applied studies of these insects. The application of CRISPR–Cas technologies to mosquito transgenesis is likely to make obsolete some aspects of transposon-based technologies except in circumstances where quasi-random insertion generation is desirable (enhancer traps, random “docking” sites for SSR, insertion-site impact analyses) (see Topic Introduction: Design and Validation of Guide RNAs for CRISPR/Cas9 Genome Editing in Mosquitoes [Lo and Matthews 2023]; Amenya et al. 2010; O'Brochta et al. 2012; Carballar-Lejarazú et al. 2013). Therefore, efficient and routine methods for transposon-mediated germline transgenesis and genomic analyses provide tools for investigations where its unique, remobilizable, insertional mutagenesis features are needed.
ACKNOWLEDGMENTS
Funding was provided by the University of California Irvine Malaria Initiative. A.A.J. is a Donald Bren Professor at the University of California, Irvine. We are grateful to Kiona Parker for providing images of transgenic An. stephensi larvae.
Footnotes
-
From the Mosquitoes collection, edited by Laura B. Duvall and Benjamin J. Matthews.