Immunizing Animals
Abstract
The traditional method for generating polyclonal and monoclonal antibodies requires the immunization of an animal. Selecting the best species of animal and getting that animal's immune system to respond to a target antigen with an antibody response are essential to obtaining good-quality antibodies and hybridomas. There are only a limited number of opportunities for a researcher to intervene to manipulate and tailor the response to a particular antigen. Here we present advice and methods for designing the way in which the antigen is presented to the immune system (i.e., the immunization protocol), including the choice of animal, the antigen dose, the use of adjuvants, the route and number of injections, and the period between injections.
USING ANIMALS
Immunizing and bleeding animals to produce polyclonal sera, or to act as donors for hybridoma fusions, is a technically straightforward and humane procedure. Healthy and well-cared-for animals are essential to obtain good antibody responses. To inject and bleed animals safely and painlessly requires skill, patience, and practical training. In most countries, these procedures and the care and maintenance of laboratory animals are governed by specific legal requirements. These regulations vary from country to country but are designed to ensure the welfare of animals, to ensure that the operator is skilled, and that the manipulation is justified. The use of animals is also clearly governed by moral considerations and is the subject of intense public debate. It is the duty of the investigator to acquire a thorough knowledge of the relevant regulations and to abide by them. The investigator must take responsibility for the animals used. All investigators should receive whatever training is required, or ensure that the procedures are performed by those who have. The local legality of any procedure described here must also be verified.
Choice of Animal
The choice of animal for immunizations is determined by four points: (1) whether polyclonal or monoclonal antibodies are desired; (2) how much serum is needed; (3) from which species the antigen is isolated; and (4) how much antigen is available.
Whether to generate a polyclonal antibody or a monoclonal antibody largely depends on its subsequent application. Polyclonal antibodies are produced from many different B cells, each secreting a unique antibody. Polyclonal antibodies react with multiple epitopes on an antigen. Monoclonal antibodies, by definition, are a single subclass of antibody originally derived from a single B cell that recognizes a single epitope on an antigen. This single B cell is immortalized into a hybridoma cell line by fusing it with a myeloma cell. Polyclonal and monoclonal antibodies have many overlapping uses, but some applications are more suited to one or the other (Tables 1, 2).
Properties of polyclonal versus monoclonal antibodies
Choice of polyclonal versus monoclonal antibodiesa
In general, polyclonal antibodies work better when bound to affinity chromatography resins for use in protein purification. They are also better as capture reagents in assays such as sandwich ELISAs. Because polyclonal antibodies are mixtures of antibodies to multiple epitopes, many antibodies bind to the target protein amplifying the signal in assays like immunohistochemistry, immunocytochemistry, immunoprecipitation, and immunofluorescence.
Monoclonal antibodies are a more uniform reagent than polyclonal antibodies because only one antibody produced, which is reactive to a single epitope on a target protein. This gives them greater specificity than a polyclonal, allowing for pairs of antibodies to be used to bind to the same target protein simultaneously, making complex experiments possible. For instance, a given cell-surface receptor can be tagged to indicate its presence with one monoclonal antibody and then have its binding site blocked by another to abrogate its function. Monoclonal antibodies have made multiplexing experiments feasible. They can be made into bispecific reagents useful for bringing different cell types together, like cytotoxic T cells and cancer cells. The specificity of monoclonal antibodies is useful for targeting particular cell types or pathways, making them of interest to pharmaceutical companies as drug products (i.e., biotherapeutics).
Which Species/Strain?
A range of vertebrate species can be used to produce polyclonal antisera. The most commonly used laboratory animals are rabbits, mice, rats, hamsters, and guinea pigs (Table 3). Typically, a single sample bleed from these animals will yield 25 mL of serum from a rabbit, 100–200 µL from a mouse, and 1–2 mL from a rat, hamster, or guinea pig. Only when large volumes of sera are required are larger animals needed. Pigs, horses, goats, sheep, and donkeys are all used commercially and can be used if large volumes of immune serum are needed.
Choice of animal
For practical reasons, rabbits represent a good choice for the routine production of polyclonal sera. They are easy to keep and handle and can be bled safely and repeatedly, and the antibodies they produce are well characterized and easily purified. With careful management, at least 500 mL of serum can be obtained from one rabbit through the course of an immunization regime. Most laboratory rabbits are outbred, and relatively little is known of the genetics of the immune response in this species. This gives them a wider range of major histocompatibility complex (MHC) class II proteins and other immune response proteins than inbred animals.
For most purposes, smaller animals such as rats, mice, hamsters, and guinea pigs are not the animal of choice for polyclonal antibody production, mainly because only small volumes of serum can be obtained. This problem can be reduced by inducing the formation of ascites in mice, which can provide up to 10 mL of ascites fluid from a single animal, and antibody titers in ascites fluids are almost as high as serum titers. However, ascites production must be justified before most Institutional Animal Care and Use committees will allow it because of the discomfort and pain it causes to the animals.
Small rodents can be used for polyclonal antibody production when only small amounts of antibody are needed or when only tiny amounts of antigen are available. Small rodents generally respond better to lower antigen doses than larger animals. For monoclonal antibody production, both mice and rats can be used. Most laboratories use inbred strains of mice and rats. Here, the genetics of the immune response have been intensively studied. Genes that affect both the quality and quantity of the antibody response occur at many distinct loci, but those associated with the MHC class II proteins are often the most important (Allen et al. 1985). If a weak response to an antigen is seen, changing the strain of animal that is immunized can help overcome the low production. Table 4 lists the MHC class II alleles of many of the standard strains of mice. A reasonable approach for dealing with the MHC class II genetic background is to immunize one of the standard strains kept in your animal facility, and if there is no response or only a weak response, then consider alternative strains. In particular, F1 hybrid mice and/or laboratory “outbred strains” such as Swiss Webster mice are often useful.
Mouse haplotypes
All immune systems display the property of self-tolerance. This actively acquired state protects the animal from autoimmune damage and can have a dominant role in determining the immunogenicity of a given antigen. Thus, at its simplest, mouse serum albumin is not immunogenic in mice but is in rabbits. In practice, immunizations should be performed in animals that are as far evolutionarily from the source of the antigen as possible. For weakly immunogenic, highly conserved proteins isolated from mammalian sources, chickens or other fowl provide a valuable alternative because their self-tolerance profile to such molecules is considerably different from that of mammals. If mice need to be used for immunizations with well-conserved antigens, NZB mice often are a good choice because they tend to make autoantibodies more readily than other strains. Knockout mice in which the gene for a specific protein (antigen) has been removed before birth offer a good alternative for high-homology target antigens. Because the animal does not express a given protein, the immune system should see it as a foreign protein and respond accordingly.
Animals from various species reach immune maturation at different times post-birth. Mice are commonly considered to be mature at 6 wk, rats at 6–8 wk, and rabbits 12 wk post-birth. It is ill advised to immunize animals before their immune systems have fully matured because tolerance can result in immunological unresponsiveness to the immunogen.
How Many Animals?
Even in genetically identical animals, a single preparation of antigen often elicits a different antibody response. When outbred animals such as rabbits are used, these differences are heightened. If the amount of antigen is not limiting, several animals should be used for any immunization scheme, and individual animals should always be screened separately. For rabbits, two animals should be used as a minimum, with three to four being preferable. For mice or other rodents, three to six is advisable.
ANESTHESIA
Anesthesia is used to sedate animals during injections or during operations where unexpected or rapid movements would endanger the animal (see Protocol: Administering Anesthesia to Mice, Rats, and Hamsters [Greenfield 2019a] and Protocol: Administering Anesthesia to Rabbits [Greenfield 2018a]). For laboratory animals, the most common anesthetics are isoflurane, carbon dioxide, sodium pentobarbital, fentanyl/fluanisone, and fentanyl/droperidol. The suggested doses for these drugs vary over a surprisingly wide range (Table 5); consult with your institutional veterinarian for the appropriate dose.
Choice of anesthesia
Isoflurane is administered by inhalation either by placing the animals (often mice or rats) in a closed container permeated with a mixture of isoflurane and oxygen vapor or (for larger animals) by using a mask. Animals sedated by isoflurane can be handled for injections or simple operations. The depth of anesthesia is easy to control and to assess. Carbon dioxide is also administered by inhalation and is useful for short periods of 1–2 min. CO2 should be mixed 1:1 with oxygen delivered either in a closed container or with a mask; a syringe barrel can be used to continue anesthesia. CO2 treatment is useful for injections, bleedings, or ascites tapping.
Sodium pentobarbital (Nembutal, Sagatal) is delivered by injection. Mice and rats are normally injected intraperitoneally (i.p.), whereas rabbits are injected intravenously (i.v.). Sodium pentobarbital can be used for operations or for more complicated injections. Fentanyl/fluanisone and fentanyl/droperidol combinations are used for the heavy sedation needed for major surgery. For the mouse and rat, injections are performed either i.p. or intramuscularly (i.m.), and for the rabbit, i.m. These injections are often preceded by an intraperitoneal injection of diazepam to potentiate the anesthetics.
To judge the depth of anesthesia, pull the animal's hind leg out straight and pinch the foot hard using the thumb and forefinger. Animals should not be operated on until the withdrawal reflex has abated.
ADJUVANTS
Nonspecific stimulators of the immune response are known as adjuvants. There are two main types: oil-based and water-based. Each type can be obtained with or without immune modulators. The judicious use of adjuvants is essential to induce a strong antibody response to soluble antigens, although they might not be required for particulate or whole-cell antigens. The action of adjuvants is not fully understood, but most adjuvants incorporate two components. One is a substance designed to form a deposit protecting the antigen from rapid catabolism. The two traditional methods of forming a deposit are to use mineral oils or aluminum hydroxide precipitates (Glenny et al. 1926). With mineral oils, such as those used in Freund's adjuvant, the immunogen is prepared in a water-in-oil emulsion. For aluminum hydroxide, the immunogen is either adsorbed to preformed precipitants or is trapped during precipitation. Alternatives to the delivery systems include liposomes or synthetic surfactants (Hunter et al. 1981). Liposomes are only effective when the immunogen is incorporated into the outer lipid layer; entrapped molecules are not seen by the immune system. The most effective of the synthetic surfactants are the pluronic polyols.
The second component needed for an effective adjuvant is a substance that will stimulate the immune response nonspecifically. These substances act by attracting and inducing phagocytic cells to raise the level of a large set of soluble peptide growth factors known as lymphokines. Lymphokines stimulate the activity of antigen-processing cells directly and cause a local inflammatory reaction at the site of injection. Early work relied entirely on heat-killed bacteria (Dienes 1936) or lipopolysaccharide (LPS) (Johnson et al. 1956). LPS is reasonably toxic, and, through analysis of its structural components, most of its properties as an adjuvant have been shown to lie in a portion known as lipid A. Lipid A is available in several synthetic and natural forms that are much less toxic than LPS, while retaining most of the better adjuvant properties of the parental LPS molecule. Lipid A compounds are often delivered using liposomes.
The two bacteria that are commonly used in adjuvants are Bordetella pertussis and Mycobacterium tuberculosis. When used as whole bacteria, they must be heat-killed before use. The immunomodulatory mediators of B. pertussis include an LPS component and the pertussis toxin. The pertussis toxin has been purified and is available commercially. M. tuberculosis is most commonly found in Complete Freund's adjuvant. The most active component of M. tuberculosis has been localized to muramyl dipeptide (MDP) (Ellouz et al. 1974). MDP is available in several forms.
The overall effect of adjuvants is dramatic, and their importance cannot be overemphasized. The depot action means that much smaller doses of antigen can be used and that antibody responses are more persistent. The nonspecific activation of the immune response often can spell the difference between success and failure in obtaining an immune response. Adjuvants should always be used for the first injections unless there is some specific reason to avoid them.
An intense drive to find potent adjuvants with more acceptable side effects has led to the production of new synthetic adjuvants. Four that are available and offer many advantages are SAF-1 (formulated by Syntex Research, Palo Alto, CA); RAS (Ribi Adjuvant System, Ribi ImmunoChem Research, Hamilton, MT, and distributed by Sigma-Aldrich, St. Louis, MO); GERBU Adjuvant (GERBU Biotechnik GmbH, Gaiberg, Germany); and Magic Mouse Adjuvant (Creative Diagnostics, Shirley, NY).
When beginning an immunization, choosing the correct adjuvant can be difficult. As a general suggestion, Freund's adjuvant should be used when small amounts of the immunogen are available. If large amounts are available or if the compound is known to be highly immunogenic, then other adjuvants can be used. Freund's or any oil-based adjuvant must never be given intravenously. If the target site (epitope) is conformational or discontinuous, the immunogen might require a hydrophilic environment to retain the conformation of the targeted site; water-based adjuvants would be a better choice for these types of antigens. If any single adjuvant has been tried and the immune response in the animal has been weak, try switching to a different class of adjuvant. An adjuvant that works well for one immunogen may not be the best choice for a different immunogen. Table 6 provides a list of adjuvants recommended for use with laboratory animals. Additional information concerning adjuvant selection can be found in Stills (2005).
Choice of adjuvant
Freund's Adjuvant
The most commonly used adjuvant for research work is Freund's adjuvant (see Protocol: Preparing and Using Adjuvants [Greenfield 2018b]). Freund's adjuvant is a water-in-oil emulsion prepared with nonmetabolizable oils. If the mixture contains killed M. tuberculosis, it is referred to as Complete Freund's adjuvant (CFA); without the bacteria, it is Incomplete Freund's adjuvant (IFA). Freund's is one of the best adjuvants for stimulating strong and prolonged responses because of the nonspecific immunopotentiation of macrophages by the surfactant and the mycobacterium. The principal disadvantage of Freund's adjuvant is that it can invoke a strong inflammatory response as well as very aggressive and persistent granulomas. For this reason, it is not used for injections of humans, and the possible side effects should be monitored carefully during antibody production in animals. To avoid the majority of the side effects, the primary injection should be given in CFA, but all boosts should be performed using IFA. The Freund's/antigen emulsion should be kept sterile to avoid Pasteurella bacterial infections, which can induce carbuncles at the injection site that could rupture and compromise the health of the animal. Because Freund's adjuvants are potentially harmful to humans, care should be taken during preparation and injection.
Ribi Adjuvant System (RAS)
Ribi adjuvants are a mixture of oil, detergent, and immunostimulator(s) (see Protocol: Preparing and Using Adjuvants [Greenfield 2018b]). A metabolizable oil, squalene, is used to minimize inflammation. Modified bacterial products have been added that have been designed to provide immunopotentiation without excessive inflammation. Trehalose dimycolate is a mycobacterial component that acts as both an immunostimulator and a surfactant, as well as aiding the antigen to bind to the oil droplets. This makes Ribi an oil-in-water emulsion, which causes less tissue damage than a water-in-oil emulsion like Freund's adjuvant. However, because Ribi uses less oil, the depot effect is reduced as well, and more frequent booster injections will be required.
Ribi adjuvants are referred to as an adjuvant system because they offer different detoxified bacterial products as immunostimulators specific for different species. For some species, this might make Ribi a better choice than Freund's. In general, Ribi is a good choice for proteins that have some hydrophobic or amphipathic properties because the adjuvant's effectiveness depends on adsorption of the antigen to the oil droplets. This can permit a better antibody response to epitopes of the native protein compared with denatured protein.
Hunter's TiterMax Adjuvant
Hunter's TiterMax is an oil/surfactant-based adjuvant that is water-in-oil based like Freund's adjuvant (see Protocol: Preparing and Using Adjuvants [Greenfield 2018b]). Unlike Freund's, it uses squalene and a synthetic nonionic surfactant (a copolymer of polyoxyethylene and polyoxypropylene) with good protein-binding capacity. The surfactant can activate complement and bind complement components that help target the antigen to follicular dendritic cells in the lymph nodes and spleen. TiterMax Gold is the latest incarnation, which works effectively in mice and rabbits. Depending on the antigen, it can work as well as or better than Freund's. The main advantage of TiterMax is that it uses copolymer-coated microparticles to form stable emulsions with less oil. This reduces the amount of inflammation at the injection site and reduces the frequency of booster injections.
Magic Mouse Adjuvant
Magic Mouse adjuvant is an aqueous suspension that has been tailor-made to induce the rapid production of high titers of antibodies in mice. The adjuvant contains immune-stimulatory CpG DNAs, which are short oligonucleotides containing unmethylated cytosine–guanine dinucleotides within a certain base context, which can be found naturally in bacterial DNA. CpG activates the innate immune system through Toll-like Receptor 9 (TLR9) found on macrophages. TLR9 triggering activates a signaling cascade leading to the production of pro-inflammatory cytokines and activation of the innate immune system. When mixed with an antigen, CpG DNA produces high titers of antigen-specific antibodies.
Because different CpG DNA sequences activate the immune systems of different animal species, Magic Mouse adjuvant is specifically designed for immunization of mice. Unlike water-in-oil emulsions, Magic Mouse adjuvant preparation does not require sonication, heating, lyophilization, or homogenization, allowing the native conformation of the immunogen to be maintained. Because Magic Mouse is aqueous based, there is no depot effect and frequent booster injections could be required. Magic Mouse is nontoxic with no adverse effects. This can be useful for generating antibodies against conformational epitopes on native antigens.
Pam3Cys-Ser-(Lys)4 Adjuvant
Pam3Cys-Ser-(Lys)4 is a lipopeptide that has been reported to be a potent stimulator of immune responses in mice and rabbits (Bessler and Jung 1992), markedly enhancing the immune response when given as a mixture with antigens. The reported side effects have been significantly less than for Freund's adjuvant while still inducing a significant antigen-specific immune response (Kellner et al. 1992). Pam3Cys-Ser-(Lys)4 can be covalently coupled with most antigens. It is a TLR2 agonist, inducing a signaling cascade effect leading to the activation of pro-inflammatory transcription factor NF-κB.
GERBU Adjuvant
GERBU adjuvant is an aqueous-phase adjuvant. This means that it lacks any depot effect, making it necessary to boost the animals frequently to obtain a high-titer response. It shows minimal inflammation at the injection sites and avoids many of the drawbacks seen with Freund's adjuvant. GERBU is mainly a colloidal suspension of cationic nanoparticles, GMDP (a glycopeptide derived from Lactobacillus bulgaricus cell walls) and cimetidine (a histamine antagonist). The nanoparticles bind the antigen and carry it to the lymphocytes, where it is phagocytized and presented to the immune system.
Aluminum Hydroxide Adjuvant
A common alternative to Freund's adjuvant is to adsorb the immunogen onto an aluminum salt (Glenny et al. 1926; see also Protocol: Preparing and Using Adjuvants [Greenfield 2018b]). Aluminum hydroxide is the most commonly used, but every aluminum salt avoids many of the more harmful side effects of Freund's adjuvants. The immunogen is either allowed to adsorb to the preformed aluminum salt or it can be trapped in the salt during precipitation. Adsorption of the protein is dependent on the pI: proteins with lower pI adsorb more strongly to the positively charged aluminum salts. When injected, the precipitate provides the depot effect.
If desired, nonspecific stimulation can be provided by adding killed bacteria, such as heat-killed B. pertussis, to the preparation. However, the addition of B. pertussis reintroduces some of the potential side effects seen with Complete Freund's adjuvant. Aluminum hydroxide adjuvants, when used without the B. pertussis stimulants, can be injected into all sites. When B. pertussis or other agents are used, intravenous injection is no longer an option.
Aluminum hydroxide adjuvants are generally much weaker than emulsion-based adjuvants. However, because of their mild inflammatory reactions, efficacy in generating memory, and fewer safety issues, they are the primary adjuvant used in humans.
DOSE OF THE ANTIGEN
Two criteria are important to consider in determining the proper dose: the optimum dose to achieve the strongest response, and the minimum dose likely to induce the production of a useful polyclonal antiserum or hybridoma fusions. Much of the injected material will be catabolized and cleared before reaching an appropriate target cell. The efficiency of this process will vary with host factors, the route of injection, the use of adjuvants, and the intrinsic nature of the antigen. The effective dose delivered to the immune system might bear little relationship to the introduced dose. Thus, descriptions of dose requirements are inevitably empirical. Ultimately the amount of an antigen required to induce a good immune response will be dependent on the individual antigen and the specific host animal. For rabbits, if a pure, soluble protein antigen is being used and is abundant, then a dose of 0.5–1 mg in adjuvant at each immunization is a sensible general recommendation (Table 7); for mice, this figure can be reduced 10-fold to 50–100 µg (Table 8). This 10-fold difference between rabbits and mice is a good general rule for determining the amount of antigen needed to produce a strong response when comparing these two animals. For rats, hamsters, and guinea pigs, a dose of 100–200 µg is sufficient (Table 9). Lower doses can be used for antigens with higher immunogenicity.
Suggested doses of immunogens in rabbits
Suggested doses of immunogens for mice
Suggested doses of immunogens for rats and hamsters
The minimum amount of antigen capable of inducing a response will depend on the nature of the antigen and on the host, but in some mouse systems, it might be possible to obtain a good response to fractions of a microgram of antigen. For rabbits, the minimum dose will be in the range of 10 µg per injection, but 100 µg per injection will be used more commonly (see Table 7). If no information is available concerning the immunogenicity of an antigen, the best advice is to perform the injection and observe the response. If antibody responses are not required following the primary injection, effective initial doses can be kept quite low. Secondary injections and subsequent boosts can be given with amounts similar to the priming injection. These recommendations are somewhat contradictory to the standard traditional suggestions for doses. Traditional suggestions propose that primary injections should use two to three times more antigen than boosts. However, most of these recommendations are based on good immunogens and in cases in which the immunogen is available in large supplies. For rare antigens, one or two injections with a low dose to prime the animals followed by a larger secondary boost have often been shown to be effective for antibody production.
FORM OF THE IMMUNOGEN
The form of an antigen is also of crucial importance (see Introduction: Selecting the Antigen [Greenfield et al. 2021]).
Soluble Antigens
Soluble protein and peptide antigens are the easiest with which to work. They can yield strong responses and good monoclonal antibodies with doses as low as 1 µg/injection. More commonly, injections are adjusted to deliver 10–20 µg. If the antigen is available in large quantities, 50 µg should be used. Except for special cases, it is seldom worthwhile to use more than 200 µg of a protein antigen per injection. Even if the antigen is not pure, the total dose should not normally exceed 500 µg. When highly conserved proteins are being used to raise antibodies, it is often necessary to modify these antigens before injection. This can be performed by covalently adding small immunogenic haptens to proteins. Modifying proteins by binding them to large immunogenic proteins such as the hemocyanins has also been shown to be an effective way of breaking T-cell tolerance. These methods are discussed in detail in Introduction: Selecting the Antigen (Greenfield et al. 2021).
Insoluble Antigens
Particulate antigens can be much better immunogens than soluble molecules. Whole cells, bacteria, and viruses are usually very immunogenic. Soluble monomeric fractions of many proteins induce poor responses. Yet, when the same protein molecules are aggregated, they induce a good antibody response. There are also many examples of weakly antigenic proteins that have been made highly antigenic by coupling them to large matrices such as agarose beads. One of the reasons for these effects is the rapid phagocytosis of particulate antigens compared with soluble material. Most soluble antigens can be made more immunogenic by coupling them chemically to beads or cells, by converting them to larger compounds by self-polymerization (e.g., with chemical cross-linkers, or by partial denaturation), or by binding them to carrier proteins. Large, insoluble antigens should not be given intravenously because they can induce embolisms, killing the animal.
The valency of an antigen can also have a strong effect on its immunogenicity. This is particularly true for large complex carbohydrates, which are much more immunogenic than simple compounds. Apparently, the repeating epitopes on these immunogens make better targets for binding to B cells. However, polymeric antigens with nondegradable backbones can paralyze the B cell by blocking its surface receptors and preventing the immunogen from being processed.
Recombinant Proteins Produced by Overexpression
Recent advances in recombinant DNA technology have simplified the production of many protein antigens. Overexpression of fusion proteins or full-length polypeptide chains using both prokaryotic and eukaryotic vectors has become routine. These proteins are often excellent antigens and can be produced in large quantities. They normally present few problems for the production of monoclonal antibodies. These proteins can be purified and injected as soluble or insoluble antigens.
Synthetic Peptides
Synthetic peptides, when coupled to carrier proteins such as bovine serum albumin or keyhole limpet hemocyanin (KLH), elicit a strong humoral response. Constructing these carrier complexes and the production of antipeptide sera are described in Introduction: Selecting the Antigen (Greenfield et al. 2021). Using peptide–carrier protein complexes for the production of monoclonal antibodies normally is done only for specific reasons. Because these peptides are relatively short, many of the advantages of monoclonal antibody specificity are lost. Monoclonal antibodies do provide two advantages over polyclonal antipeptide sera. The first is that the source of the antibodies will be unlimited, and the second is that monoclonal antibodies can be more useful in immunoaffinity purifications. Like all immunizations using peptide antigens, the major difficulty will be in preparing antibodies that will also bind to the native protein.
Live Cells
Several studies have used live cells as immunogens for generating antibodies to surface antigens. Except in unusual circumstances, injections of cells should not include live bacteria or yeast. Although mice are normally capable of killing and clearing bacteria and yeast infections, the possibility of infecting an entire mouse colony is too great to risk these types of injections.
Although large numbers of hybridomas have been prepared to surface antigens of mammalian cells, these antibodies can be of low affinity, and care should be taken to ensure that the immune response includes antibodies that will be useful in subsequent studies. When raising antibodies to live tumorigenic cells, it is easy to pass the cells as tumors and thereby eliminate any activity against tissue culture reagents, including proteins in bovine serum.
Nucleic Acids
Nucleic acids normally are not good target antigens. Antibodies to them usually are raised against small haptens bound to carrier proteins. Because nucleic acids are weak antigens, it is particularly important to test sera for antibodies that will work in all assays for which the monoclonal antibodies are being raised.
Carbohydrates
Simple carbohydrates usually are weak immunogens. These compounds should be coupled to carrier proteins. Large complex carbohydrates (>50,000) will induce a moderate response, but often without a secondary response. High doses readily induce tolerance; thus, the injected amount should be controlled carefully. These immunogens are best injected as a portion of a larger particle, such as a bacterial cell wall or equivalent. Coupling these larger carbohydrates to carrier proteins can be beneficial. For glycoproteins, the polypeptide backbone can function as an effective carrier.
ROUTES OF INJECTION
The route of injection is guided by three practical decisions: (1) the volume to be delivered, (2) the buffers and other components injected with the immunogen, and (3) how quickly the immunogen should be released into the lymphatics or circulation (for a review of lymphatic tissues, see Allen [1967]). For rabbits, large-volume injections normally are given at multiple subcutaneous sites. For mice, large volumes are only practical with intraperitoneal injections. If adjuvants or particulate matter are included in the injection, the immunogen should not be delivered intravenously. If slow release of the inoculum is desired, the injections should be performed either intramuscularly or intradermally (i.d.). For immediate release, use intravenous injections. There are numerous reviews and summaries on routes and techniques of injections; two useful general sources are Herbert and Kristensen (1986) and Poole (1987).
Most injections for hybridoma production are performed in female mice because they are somewhat easier to handle than male mice. Before beginning an immunization, contact your local safety and animal committees for advice on animal care and handling, local regulations, and proper procedures for immunization. Table 10 and Table 11 summarize the potential routes of introducing an antigen into mice and rabbits, respectively.
Routes of injection for mice, rats, and hamsters
Routes of injection for rabbits
For most injections, disposable syringes are best. Plastic syringes are appropriate for all injections except when using Freund's adjuvant, in which case, disposable glass syringes are recommended. Syringes can be purchased with several different tips. For most work, the Luer tip is fine, but use the Luer lock for samples that are viscous. After removing the syringe from its container, push the plunger in completely to loosen the seal that has formed between the plunger and the barrel.
For removing samples from a closed vial through a septum, fill the syringe with air to approximately the total volume to be removed from the vial. Rub the top of the septum with an alcohol-soaked pad. Insert the needle through the septum, and inject the air into the vial. Lift the vial vertically above the syringe. Adjust the point of the needle until it is within the liquid in the vial. Withdraw the plunger and depress several times to wet the inside surfaces of the syringe and to dislodge any air. Withdraw the plunger to the appropriate amount. Remove the needle from the vial. Check to be certain that there is no air in the needle by depressing the plunger until a drop is seen at the tip. The samples are now ready for injection.
To remove samples from an open vial, depress the plunger completely. Place the needle in the sample, and withdraw slightly more than the total volume to be injected. Remove the syringe from the sample, and place it in a vertical position with the needle topmost. Withdraw the plunger until an air space can be seen in the barrel. Dislodge any air bubbles trapped on the side of the syringe by tapping the barrel sharply with a finger or with any object. Depress the plunger gently until a drop is seen at the tip of the needle. The samples are now ready for injection.
Subcutaneous Injections
Subcutaneous injections (s.c.) are used widely to immunize laboratory animals, especially rabbits (see Protocol: Routes of Antigen Injection in Rabbits [Greenfield 2018c] and Protocol: Routes of Antigen Injection in Mice and Rats [Greenfield 2019b]). Injections can include both particulate immunogens and/or adjuvants. Injected material will drain quickly into the local lymphatic system and will become concentrated in the lymph nodes closest to the injected sites. Larger animals such as rabbits normally are given multiple subcutaneous injections on the back, whereas mice are injected on the back of the neck or on one or both sides in the groin. Each injection should be relatively small, often 50–100 µL for mice, up to 400 µL for rats, and up to 800 µL for rabbits. Large injection volumes containing Freund's adjuvant should not be injected into one site because they can cause severe granulomas. The inoculum is far better delivered by multiple (up to 10 sites for a rabbit) doses in separate sites. Anesthesia is normally recommended for larger animals, but is not normally required for mice. It is used only to allow easier handling during injections.
Subcutaneous injections are used to deliver soluble or insoluble antigens into a local environment that is a good site of lymphoid draining. Maximum volumes for subcutaneous injections are about one-fifth the maximum used for intraperitoneal (i.p.) injections (100 µL compared with 500 µL). Subcutaneous injections normally are performed at more than one site to help ensure that the antigen is detected.
Like peritoneal injections (see below), subcutaneous injections that do not use adjuvants normally are used for delivering live cells to the mouse. This route is often used for tumorigenic cells.
Protein antigens immobilized on nitrocellulose often make exceptionally good immunogens. This is probably the result of their slow release from the paper, thus behaving somewhat like an adjuvant. Not all antigens show increased immunogenicity using this methodology, but some do. The antigen is bound to paper and is implanted on the back of the mouse's neck, a location that makes it difficult for the mouse to disturb the surgical clip (Protocol: Immunizing Mice and Rats with Nitrocellulose-Bound Antigen [Greenfield 2019c]).
Intramuscular Injections
Intramuscular injection (i.m.) is one of the routes of inoculation used to produce a slow release of an antigen (see Protocol: Routes of Antigen Injection in Rabbits [Greenfield 2018c] and Protocol: cDNA Immunization of Mice, Rats, and Hamsters [Greenfield 2021a]). The inoculum is deposited directly into the muscle tissue, and the antigen is seen as it drains into the nearby interstitial spaces. The inoculum will then drain into the local lymph nodes. Because of the difficulty of injecting into the muscle tissue of smaller rodents, intramuscular injections are not recommended for mice. Rabbits can be injected with volumes up to 0.5 mL, and both adjuvant and particulate inoculi can be used.
Intradermal Injections
Intradermal injection (i.d.) is used quite commonly for the immunization of larger animals (see Protocol: Routes of Antigen Injection in Rabbits [Greenfield 2018c]). Like intramuscular injections, intradermal injections are used to give a very slow rate of release of the immunogen. The inoculum is injected between the layers of the skin and is slowly absorbed into the body. Intradermal injections can be used with particulate- and adjuvant-containing inoculi.
Only small injection volumes can be used; thus, intradermal injection is only suitable if the antigen is available in a concentrated form. It is normal practice to deliver the injection over multiple sites. The procedure requires more skill than other injections. It is technically difficult to administer with small rodents and thus is seldom used for mice.
Intravenous Injections
For most laboratory animals, intravenous injection (i.v.) is a practical method of delivering a secondary or subsequent boost (see Protocol: Routes of Antigen Injection in Mice and Rats [Greenfield 2019b] and Protocol: Routes of Antigen Injection in Rabbits [Greenfield 2018c]). It is seldom used for a primary injection. Antigen delivered directly into the bloodstream will be processed rapidly by the reticuloendothelial system, primarily in the liver, lungs, and spleen. Direct intravenous injection carries serious risk to the host animal because it can readily induce pulmonary embolisms or lethal anaphylactic shock in sensitized animals. Any toxic material present in the inoculum, such as bacterial endotoxins, is particularly dangerous when delivered i.v. It is not safe or appropriate to use Freund's adjuvant with intravenous injections. Physiological buffers and salt conditions should be used if at all possible. Detergent concentration should never exceed 0.1% for ionic detergents or 0.2% for nonionic detergents.
Intravenous injections can be used for two purposes. When immunizing mice, their main use will be to deliver the final boost just before a hybridoma fusion. Intravenous injections are also useful to ensure that the antigen is seen by the immune system. A rapid and strong response can be expected because the antigen will be collected quickly in the spleen, liver, and lungs. The antigen will be processed quickly, and no continued release of the antigen into the immune system can be expected. Consequently, these types of injections produce a short-lived response.
Anaphylactic Shock
Anaphylactic shock is one of the types of antibody-mediated hypersensitivity responses (i.e., allergies). When the injection of an immunogen induces a strong IgE response, the animal is often sensitized to the antigen and will develop an allergic response on subsequent exposure. When the injections are given s.c., i.d., or i.m., although an allergic response might develop, the animal is normally in no danger. However, when the injection is given i.v., anaphylactic shock (systemic anaphylaxis) can ensue. Immediate hypersensitive responses are characterized by the release of histamine and vasoconstriction, followed by dilation of peripheral blood vessels. In most cases, if left untreated, the animal will die from suffocation. When using intravenous injections, a supply of epinephrine must be handy to guard against death from anaphylactic shock. Epinephrine is given i.v. Consult your institutional veterinarian for the appropriate dose.
Intraperitoneal Injections
Direct injection into the peritoneal cavity (i.p.) is particularly easy and appropriate in small rodents (see Protocol: Routes of Antigen Injection in Mice and Rats [Greenfield 2019b]). Antigens injected into the peritoneum will drain into the thoracic lymphatics and then to the vena cava. Particulate antigens or antigens in an adjuvant can both be given safely by this route. Repeated injection of Complete Freund's adjuvant by this route will induce granulomas and fibrous adhesions within the peritoneal cavity that can make subsequent disruption of the spleen difficult for hybridoma production. If the spleen is to be collected for hybridoma fusion (see Protocol: Harvesting Tissue from Immunized Mice, Rats, and Hamsters [Greenfield 2017a]), then the intraperitoneal injection should be performed on the right side. It can also cause the production of ascites fluid and even plasmacytomas in some animals. Ascites fluid induction can be an extremely useful way to obtain large quantities of polyclonal mouse antisera. It can be enhanced by a carefully timed injection of plasmacytoma cells.
Intraperitoneal injections are the most commonly used method for introducing antigens into mice. Because of the large volume of the peritoneal cavity, the volume of the immunogen can be larger for i.p. injections than for other sites. In addition, because the injections do not deliver the antigen directly into the blood system, particulate antigens can be used. Intraperitoneal injections are not often used for inoculating rabbits because of their larger size. Other routes such as subcutaneous or intramuscular are normally used.
Intraperitoneal injections can also be used to deliver live cells into the peritoneal cavity. These immunizations normally are used to introduce live mammalian cells into mice and prepare anti–cell-surface antibodies. In general, adjuvants should not be used.
One excellent method to increase the chances of an antigen being phagocytosed is to bind it to beads. Protein antigens can be bound by free amino groups to any of a number of agarose or polyacrylamide beads. The methods for coupling are discussed in Topic Introduction: Selecting the Antigen (Greenfield et al. 2021). After coupling, the beads are injected intraperitoneally with adjuvant as described in Protocol: Routes of Antigen Injection in Mice and Rats (Greenfield 2019b). These preparations should never be injected intravenously because the chance of forming embolisms is too great.
Protein antigens can be bound to nitrocellulose and injected or implanted into the peritoneum. Two approaches can be used. If the entire piece of nitrocellulose will be implanted, the subcutaneous route is suggested (Protocol: Immunizing Mice and Rats with Nitrocellulose-Bound Antigen [Greenfield 2019c]). For intraperitoneal injections, the nitrocellulose should either be fragmented or dissolved.
Injections into Lymphoid Organs
Injections directly into a lymphoid organ offer some strong theoretical advantages over other injection routes (e.g., see Sigel et al. 1983; Nilsson et al. 1987). The total dose of the immunogen is concentrated in one region that is specialized in dealing with it. The major difficulties are that the injection is technically more difficult than other injections, often requiring an operation, and many of the advantages of adjuvants are lost because they must be avoided because of their harmful side effects in the delicate lymphoid organ. As better adjuvants become available, some of these side effects might be less worrisome. However, the technical difficulties in delivering the immunogens are still severe, particularly for novices. Two compromises are to inject into the lower region of the leg or to mark draining lymph nodes with dyes to make them easier to visualize during surgery.
These are injections for the more specialized delivery of antigens. They are often appropriate for small amounts of antigen and particularly for secondary or subsequent boosts. In theory, these types of injections can be the best methods for giving a final boost, but because they demand more skill, they are not in common use. The two most frequently used sites of injection are the footpad and the spleen. In general, footpad and spleen injections should be performed only for highly specialized antigens.
The lower leg of a rabbit or rodent has some advantages for the injection of an immunogen. In general, injections into the lower region of the foot drain into the popliteal gland near the back of the knee or into the inguinal gland in the groin. If having the immunogen drain into lymph nodes is insufficient, an injection can be targeted directly to the lymph node by injecting a dye into the region between the toes. This will stain the draining lymphatics within 5–30 min (in mice) and can be used as a guide in locating the nodes (Harrell et al. 2008). After the dye injection, an operation can be performed to locate the appropriate lymph node for immunization. Injections are performed with one of the non-oil-based adjuvants or in phosphate-buffered saline alone. This procedure is tolerated better in larger animals like rats or rabbits than in mice.
Footpad injections are common sites of injections in some countries but are banned as inhumane in others. Unless there are particular reasons for performing injections into this site, we recommend using other routes. The injections should never be performed in the major weight-bearing regions of the pad. Often inflammation and swelling occur at the injection site, leading to the animal being unable to bear weight on its injected foot, sometimes resulting in lameness. In addition, boosts given in the footpad should never contain Complete Freund's adjuvant. Always use anesthetics for footpad injections.
An alternative to footpad immunizations for targeting draining lymph nodes is hock immunizations. Hock immunizations are performed in the lateral tarsal region just above the ankle. Hock immunization has all the advantages of footpad immunization for providing a strong immune response, draining into the same lymph nodes, but with significantly less impairment of mobility (see Protocol: Routes of Antigen Injection in Mice and Rats [Greenfield 2019b]).
Boosts
An increase in B cells bearing surface antibodies specific for the inoculated antigens is first detected 5–6 d after the primary injection of antigen. Antibody is usually detected in the serum from around 7 d after the injection and persists at a low level for a few days, typically reaching peak titer around Day 10. Primary responses often are very weak, particularly for readily catabolized, soluble antigens. In many cases, efficient priming can take place in the absence of detectable antibody production; thus, for practical purposes, it will not be of much value to monitor this response.
A delay is required before reintroducing the antigen into a primed animal. A minimum of 2 or 3 wk is recommended, but there is no penalty in leaving much greater intervals. Mice and rabbits will remain effectively primed for at least a year after receiving the first injection.
The response to the second injection of the same antigen is dramatically different. The number of B cells bearing antigen-specific cell-surface antibodies increases exponentially after the secondary injection, reaching a peak between Days 3 and 4. Antibodies in the serum are also detectable at this time, but peak levels are usually achieved around Days 10–14. Typically, high levels of antibody persist for ∼2–4 wk after the second injection.
The response to the third and subsequent injections broadly mirrors that of the secondary injection. Higher titers of antibody are reached, but, more importantly, the nature and quality of the antibodies present in the serum change. These changes are known as the maturation of the immune response and have considerable practical importance because they yield high-affinity antibody preparations. The intervals between secondary, tertiary, and subsequent injections can also be varied but usually need to be extended to allow the circulating level of antibody to drop enough to prevent rapid clearance of the newly injected antigen.
For rabbits, injections are normally given every 4–6 wk. One common error in immunizing rabbits is to boost every 2 wk. Although this will continually re-expose the animal to the immunogen, the effective size of the boost will be reduced by the circulating antibody. For mice, secondary and subsequent boosts should be spaced at least at 3-wk intervals.
Repetitive Immunizations at Multiple Sites (RIMMS)
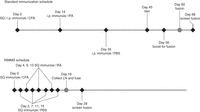
Kilpatrick et al. (1997) developed an immunization protocol that capitalizes on the rapid hypermutation and affinity maturation events that occur in secondary lymphoid tissues such as lymph nodes (see Protocol: Repetitive Immunization at Multiple Sites (RIMMS) of Mice, Rats, and Hamsters [Greenfield 2020a]). Here B lymphocytes are localized and respond more quickly to antigenic challenges. The basic RIMMS immunization protocol requires that mice be immunized five times over eleven days in several subcutaneous sites proximal to draining lymph nodes. A fusion is performed on Day 13 (Fig. 1).
Comparison of the timelines for standard immunizations versus RIMMS (repetitive immunizations at multiple sites). (SQ) Subcutaneous; (i.p.) intraperitoneally; (CFA) Complete Freund's adjuvant; (IFA) Incomplete Freund's adjuvant; (PBS) phosphate-buffered saline; (LN) lymph nodes.
The RIMMS protocol relies on immunization at sites draining into lymph nodes and the use of a myeloma partner cell that had been stably transfected with Bcl-2, the founding member of the family of apoptotic regulatory proteins. Bcl-2 plays an important role in lymphoid selection processes, including the establishment of regulation of memory B cells, maintenance of self-tolerance, preventing the expansion of pathogenic B-cell populations, and rescuing cells from apoptosis. Many B cells that do not have sufficient levels or self-reactive surface immunoglobulin fail to receive a survival signal and die. Having the Bcl-2 present in the myeloma fusion partner can enhance their survival.
The RIMMS method works mainly through the innate branch of the immune system. For this reason, it is likely to respond to conformational epitopes better than classical immunizations, which use the adaptive branch of the immune system in which antigens are phagocytized by antigen-presenting cells and presented as fragments to B cells. RIMMS can also offer more epitope diversity in response to a particular antigen, although antibody affinity might be less than with classical immunizations (Table 12).
Classical versus RIMMS immunization protocols
Genetic (Naked DNA) Immunization
Since first described in the early 1990s, genetic immunization has been growing in popularity (see Protocol: cDNA Immunization of Mice, Rats, and Hamsters [Greenfield 2021a]). Simply put, genetic immunization is introducing a gene in the form of cDNA directly into an animal, allowing it to be translated by the animal into protein, which can then stimulate an immune response. The protein is expressed in native conformation with posttranslational modifications that are not always achievable with recombinant protein expression, facilitating presentation of conformational epitopes and hard-to-express recombinant proteins. Genetic immunization has been successful in generating antibody responses to difficult protein targets such as G-protein–coupled receptors (GPCRs), ion channel proteins, and other multiple membrane-spanning proteins.
Genetic immunization is most effective for generating antibodies where the cDNA encodes for secreted or cell-surface proteins to make them accessible to the immune system. The route of cDNA immunization is either through the skin (binding to gold particles and using a gene gun; Tang et al. 1992; Sato et al. 1996), the quadriceps muscle (Belperron et al. 1999; Boyle and Robinson 2000), or intravenously (Bates et al. 2006). High-affinity antibodies are favored because the proteins are expressed at low levels and are constantly present for presentation to the immune system. In addition, several months can be saved obtaining sufficient amounts of properly folded, soluble recombinant protein or high-expressing transfected cells.
Genetic immunizations can be enhanced by adding immunomodulatory sequences to the cDNA to augment adjuvanticity or activate the immune system, such as those encoding for immunostimulatory sequences, cytokines (Krieg et al. 1995), or ligands that bind antigen-presenting cells (Boyle et al. 1998; Azevedo et al. 1999). It has also been reported that in vivo electroporation can improve the efficiency in which the cDNA is delivered (Chen et al. 2005).
Deciding to Boost Again or to Fuse
Three factors will influence the decision to proceed with the production of monoclonal antibodies, all related to the quality and strength of the immune response. First is whether the antibodies recognize the antigen of interest. This is the most straightforward of the factors and the simplest to determine. The second is a complicated set of properties of the antibodies themselves and the strength of the immune response. These properties are manifested as different titers of antibodies and different affinities of the antibody for the antigen. The third factor is the appearance of spurious antibody activities against unrelated antigens.
In many cases, the tests will be relatively easy and the interpretation apparent. First, the sera should be checked for antibodies that bind to the immunogen itself. For example, if a purified antigen is used, sera could easily be tested for activity in a simple antibody capture assay, or if whole cells are used, then testing for binding to the cell surface should be performed first. However, if the monoclonal antibodies will be used for tests other than these simple assays, test bleeds should be checked in assays that resemble, as closely as possible, the tests for which the antibodies are being prepared. For many antibodies, the most useful test will be immunoprecipitation of the antigen. This assay is easy when only testing a few samples, and it will identify antibodies that will be useful in a large number of tests that depend on binding to the native antigen. If, however, the antibodies will be used extensively in immunoblot analyses, in immunohistochemical staining, or in other tests in which many antibodies might fail to work, these tests should be run as well.
Second, sera should also be monitored for the concentration of specific antibodies by titering the test bleeds in the appropriate assays. As the immune response matures, higher levels of specific antibodies will be found. However, higher levels of antibodies do not necessarily mean higher affinities. If high affinity is crucial to the intended use of the antibody, the sera should be titered and compared in assays that are sensitive to antibody affinity, such as immunoprecipitation.
The third factor to consider is the appearance of antibody activities against extraneous antigens. This response could be directed against other antigens in your preparations or might be a response to other antigens in the mouse's environment, including invasion by a pathogenic organism. If the mouse is ill, do not proceed with hybridoma construction. Isolate the mouse in a separate cage and allow it to recover before continuing. If a particularly valuable antigen is being used, more care and veterinary help might be needed. If the antibody activities are to contaminating antigens in the immunogen, a decision must be made whether to proceed. In general, making monoclonal antibodies against complex and multicomponent antigens is a very useful way of isolating specific immunochemical probes, particularly when the antigen is difficult to purify further. However, if the response against the other antigens continues to increase without a concomitant strengthening of the response to the desired antigen, other approaches might need to be taken. Either other mice should be tested (other individuals or other strains) or the antigen might need to be purified further before proceeding.
Hyperimmunization
Polyclonal antibodies are often raised by hyperimmunizing animals. Hyperimmunization refers to injection schedules in which animals repeatedly are boosted with the same antigen. The resulting antibodies have many useful properties. For example, class shift sera from primary injections contain a substantial proportion of IgMs, whereas sera from hyperimmunized animals contain mostly IgG antibodies. Hyperimmune sera often have higher levels of IgG, a substantial part of which is specific antibody. Levels of 1 mg/mL antigen-specific IgG are possible. This is equivalent to 10% of the total IgG content of serum.
Affinity Maturation
The average affinity of antibodies for an antigen increases with repeated injections. This change continues through multiple rounds of immunization. Antibody affinity affects the sensitivity of many immunochemical procedures.
The basis for affinity maturation is fairly well understood in terms of clonal selection and somatic mutation. In the presence of limiting amounts of antigen, those B cells with the highest-affinity antigen receptors will compete most successfully for antigen. Thus, clones of B cells secreting high-affinity antibodies will be selected for proliferation. This process is extended by somatic mutation of variable-region genes, and under continuous selective pressure, higher-affinity antibodies will result. Repeated injection of low doses of antigen is often best to select for sera of high affinity, although these sera might not contain concentrations of antibody as high as those elicited by larger doses.
Clonal Dominance
The selection of high-affinity clones can also affect the quality of the response in other ways. A common finding when immunizing with large proteins is that the response becomes less diverse with repeated injections. A few clones secreting antibodies to a limited number of epitopes will tend to dominate the subsequent stages of hyperimmunization protocols. Because the process that selects these clones involves several random factors, variation is often seen from animal to animal. One way to avoid this problem is to immunize multiple animals and to monitor test bleeds regularly.
SAMPLING SERUM
After an injection, samples of serum are taken to check the production of specific antibodies (see Protocol: Sampling and Preparation of Mouse and Rat Serum [Greenfield 2017b] and Protocol: Sampling and Preparation of Rabbit Serum [Greenfield 2018d]). Comparing the titers of antibodies isolated after successive injections allows the antibody response to be monitored. Often the first test bleeds are taken before the immunizations begin, to prepare a suitable control antibody for future tests. Although test bleeds following primary injections can be collected, they normally do not produce useful antibodies because these antibodies typically have lower affinities than subsequent samples.
Test Bleeds
Except in unusual circumstances, it is seldom worthwhile to fuse antibody-secreting cells from animals that do not have a usable titer of antibodies in their serum. Periodic test bleeds collected from immunized animals should be checked for the desired antibodies (Table 13). Tests are run on small batches of serum prepared from tail bleeds of immunized mice.
Blood sampling methods
The test bleed will yield small samples of polyclonal sera. These sera should be tested in assays that will detect the presence of antibodies specific for the antigen. These tests are discussed in detail in Introduction: Immunoblotting (Litovchick 2020), Introduction: Immunoprecipitation (DeCaprio and Kohl 2020), Introduction: Immunoassays (Kohl and Ascoli 2017), Introduction: Cell Staining (Rodig 2021), and Introduction: Antibody Screening Using High-Throughput Flow Cytometry (Duensing and Watson 2018).
To make appropriate comparisons in these tests, two practical matters need to be considered. First, the test bleeds should always be titered to monitor the development of the response. The appropriate dilutions will depend on the strength of the response and on the type of assay, but, in general, 1:5 or 1:10 dilutions will be satisfactory. Second, the proper negative control should be another polyclonal serum and not an unrelated monoclonal antibody. Most often, this negative control will be serum collected either from an uninjected animal or from an animal that has been boosted with an unrelated antigen. Although it is not always necessary, using serum from a test bleed collected before immunization of the animal is the best negative control. These bleeds are known as preimmune sera.
Beginning with the first test bleeds, it is essential to mark the mice so that the immune response can be monitored in individuals. There are several methods that are currently used to identify mice. These include ear punches, toe clips, and tail markings. If your animal facility has a standard method, consult them for the proper codes. If not, an acceptable method that is not harmful to the mice is to color the toes of their hindlegs with an indelible marker. This procedure is relatively easy, and, because the marks are on the back legs, the mice do not seem to work as hard to remove the markings as on other sites. Even so, the marks need to be reapplied twice a week.
If there is a large difference between the responses in individual mice, it might be worthwhile to isolate individual mice in separate cages to ensure that the proper mouse is given the final boost.
Samples of the serum should be taken 7–14 d after an injection. This timing will correspond with the peak of antibody titers for most injection routes. Normally, small samples are collected until the desired antibodies are detected and the levels have reached acceptable levels. Test bleeds normally are performed from the marginal ear vein for rabbits, from the tail vein for mice, or from the saphenous vein of rats. Retro-orbital bleeds and submandibular bleeds are other common sites for obtaining serum samples to assess antibody titers. These sites are used because they are easily accessible and do not have high numbers of nerve endings. Taking serum samples should be relatively painless for the animal, considerably less painful than giving blood is for humans. For rabbits, 5–10 mL can be collected conveniently, and this will provide more than enough serum for most tests. For mice, rats, and hamsters, 200–300 µL is the maximum amount for most test bleeds because of the smaller blood volume in these animals.
Test bleeds normally are assayed against the immunogen itself by one of the techniques described in Introduction: Immunoblotting (Litovchick 2020), Introduction: Immunoprecipitation (DeCaprio and Kohl 2020), Introduction: Immunoassays (Kohl and Ascoli 2017), Introduction: Cell Staining (Rodig 2021), or Introduction: Antibody Screening Using High-Throughput Flow Cytometry (Duensing and Watson 2018). When assessing the quality of the sera, be sure to test the antibodies in assays that resemble as closely as possible the techniques for which the antibodies are being raised. The sera should be compared with pre-bleed and other test bleeds in titrations to determine the strength of the antibody response. Comparing titrations of various test bleeds will give an accurate measure of the course of the immunization and will provide a good measure of the correct time to begin collecting large volumes of serum.
Once a good titer has developed against the antigen of interest, regular boosts and bleeds are performed to collect the maximum amount of serum. For rabbits, boosts should be spaced every 6 wk, and serum samples of 20–40 mL should be collected ∼10–12 d after each boost. For rats and hamsters, boosts should be spaced every 2–3 wk, and serum samples of 400–500 µL should be collected 10–12 d after each boost. For mice, boosts should be spaced every 2–3 wk, and serum samples of 200–300 µL should be collected 10–12 d after each boost.
SERUM PREPARATION
Blood collected from immunized animals has two components: a cellular component (red blood cells, lymphocytes, macrophages, platelets) and a liquid component (plasma) in which the cells are suspended. Plasma makes up ∼60% of the total blood volume. It is mostly composed of water (90% by volume) but also has glucose, clotting factors, mineral ions, hormones, immunoglobulins, carbon dioxide, and other proteins/metabolic waste products dissolved within it.
Serum is collected from clotted blood or plasma without the fibrinogen and other clotting factors. Serum contains fewer proteins than plasma and is preferred for use in many clinical assays because some of the proteins in plasma or the addition of anticoagulants can interfere with the test results.
Once blood is collected, it should be allowed to clot for at least 1 h at room temperature then cooled for 1 h to 4°C. Centrifuge the cooled blood to separate the cells from the serum. This should be performed as soon as the blood is completely clotted to avoid hemolysis of the red blood cells and discoloration of the serum by the released hemoglobin. The serum can then be stored at 4°C for short periods or frozen.
EXSANGUINATION
Exsanguination is the recommended method for the collection of large volumes of serum from an animal that will be sacrificed. Consult your animal handlers for proper methods and instructions on the techniques.
INDUCING ASCITES FLUID IN MICE
Most strains of mice can be induced to produce ascites fluid by repeated injection of Complete Freund's adjuvant. In BALB/c mice, this process can be improved by injecting myeloma cells into the immune animal (see Protocol: Induction of Ascites in Mice [Greenfield 2021b]). Up to 10 mL or more of ascitic fluid can be produced by each mouse. Because these fluids contain roughly the same level of specific antibody as the animal's serum, they provide a valuable source of polyclonal antibody and can provide an alternative to using larger animals for antibody production. The induction of the ascites must be timed to coincide with the presence of high levels of specific antibody production.
Ascites have also been used as a method for monoclonal antibody production from hybridoma lines. Although this method has long been a gold standard, consistently yielding high amounts of antibody, it is considered to induce unreasonable pain and distress in animals. Because there are many alternatives for monoclonal antibody production available, unless a particular hybridoma line has a well-documented issue with current in vitro production methods, most animal use committees will not allow ascites antibody production.
CLOSING COMMENTS
There are several points that are important in designing an immunization regime that will produce the appropriate polyclonal and monoclonal antibodies:
-
Choose the appropriate animal or strain for the desired antibody. Important points to consider are (1) tolerance, (2) polyclonal or monoclonal, (3) amount of antigen available, (4) amount of serum required (for polyclonal antibody), and (5) specific properties (including ease of purification) of the resultant antibodies.
If no preference in the choice of animal is dictated, then start the immunizations in female BALB/c mice or Lewis rats (6 wk old) for monoclonal antibodies. In general, monoclonal antibodies are made in mice or rats because they are less expensive to maintain, easier to handle, and will respond to lower antigen levels than other laboratory animals. BALB/c × BALB/c hybridomas can be grown as ascites in BALB/c mice. This can be valuable both in the production of large quantities of monoclonal antibodies and in the eradication of contaminating microorganisms from cultures of hybridoma cells grown in vitro. Modern in vitro methods for monoclonal antibody production allow for the option of using other strains of mice or different species (rats, hamsters, guinea pigs, or rabbits).
-
Individual animals, even from the same genetic background, will often respond to identical antigen preparations in completely different ways. Therefore, immunizing more than one animal is a major advantage. In addition, because laboratory animals occasionally die, starting immunizations with several animals can save valuable time.
-
Hyperimmunization (multiple immunizations with the same antigen) will yield antibodies with higher affinity for the antigen, especially when the immunizations are widely spaced over a period of weeks to months. However, multiple immunizations will not continue to increase the number of epitopes that are recognized.
-
Using a standard or RIMMS protocol can result in antibodies with different reactivities. The RIMMS protocol works best with soluble antigens and targets immune cells of the innate immune system. This makes this protocol good for conformational epitopes or when a greater epitope spread is desired. Standard immunizations target the adaptive immune system, resulting in higher-affinity antibodies generated to more dominant epitopes.
-
Except in unusual circumstances, when making monoclonal antibodies, do not start the fusion until the serum from the test bleed contains antibodies with the desired specificity. This might mean extensive testing of the serum in a number of assays, but do not expect to recover antibody activities from the fusion that are not found in the test serum.
-
Spleens from reactive animals not chosen for fusion can be collected, teased apart, and frozen as a backup. There will be some loss of viable cells in the freeze/thawing process.
-
If the animal responds weakly or not at all, consult Introduction: Selecting the Antigen (Greenfield et al. 2021) for suggestions.
Selecting an Immunization Protocol
The way that an antigen is presented to the immune system and the frequency with which it is introduced can make a significant difference between a good response and a poor one (see Protocol: Standard Immunization of Mice, Rats, and Hamsters [Greenfield 2020b], Protocol: Standard Immunization of Rabbits [Greenfield 2020c], Protocol: Repetitive Immunization at Multiple Sites (RIMMS) of Mice, Rats, and Hamsters [Greenfield 2020a], Protocol: Subtractive Immunization for Mice, Rats, and Hamsters [Greenfield 2020d], Protocol: Decoy Immunization for Mice, Rats, and Hamsters [Greenfield 2021c], Protocol: Adoptive Transfer Immunization of Mice [Greenfield 2021d], and Protocol: cDNA Immunization of Mice, Rats, and Hamsters [Greenfield 2021a]). As described above, there are many different forms that an antigen can take. A few of these forms limit the route of immunization and/or the types of screening assays that can be used. The choice of immunogen, the species of animal selected, whether to use an adjuvant, the route of immunization chosen, and the immunization schedule (timing) are all interdependent on each other. It is also important to understand the purpose the antibody is going to fulfill. Once all these factors have been examined, an immunization protocol can be established.
Deciding on the best immunization protocol is an art. Just because one specific protocol works well for one antigen does not mean that it will work on another, even if they are chemically similar. Changes in the antigen dosage or using a particular adjuvant can have either a positive or a negative influence on the immune response. Table 14 highlights some of the advantages and disadvantages of the immunization methods.
Selection of immunization protocol
Devising an Immunization Protocol
It is always helpful to know what the intended use of the antibody will be. If the antibody will be used for western blotting, a synthetic peptide or recombinant protein could be used as an immunogen because the protein will be denatured in the western blot. If the antibody will be used to precipitate a native protein from cell lysates before running the western blot, then a correctly folded recombinant protein, transfected cell, or cDNA could be the immunogen of choice. Here, as with antibodies used in flow cytometry or immunofluorescence, the conformation of the antigen is important. When a blocking antibody is sought, conformation of the antigen will be crucial.
Next, it helps to know something about the target protein itself. This is often referred to as bioinformatics analysis. Are there any regions of the protein that are more hydrophilic? Are these areas exposed on the surface of the protein? Will an immunogenic peptide or the recombinant protein be soluble? Is the chosen peptide similar to regions in other irrelevant proteins that could lead to the antibody being nonspecific? What is the biology of the target protein? Will the antibody affect the health of the animals if they have it in their bloodstreams? The answers to these questions will help define what form the immunogen will need to be.
Once the form of the immunogen is settled on, it is important to determine if it needs to be modified before immunization. This may mean conjugating a peptide to one or more carrier proteins like KLH or ovalbumin. Will carbohydrates be important? Bacterial production systems do not make recombinant proteins with carbohydrates on them. If these are necessary for proper charge or conformation of the target protein, a baculovirus or mammalian expression system may need to be used. If the target is an integral membrane protein, the cell membrane may be significant for its structure. This might require cDNA or a transfected cell to be the form for the immunogen. Is an adjuvant necessary or helpful? Some immunogens are in buffers that can be toxic to the animals. Using an adjuvant will allow it to be slowly introduced in small amounts at a time, making it more tolerable.
The chosen species of animal needs to be addressed next. Polyclonal antibodies are usually generated in rabbits unless very large amounts of serum are required. Most monoclonal antibodies are made in mice unless the target protein is of murine origin, in which case, rats are used. If there are homology issues among human, mouse, and rat, then hamsters, guinea pigs, or rabbits are chosen. Chickens are used if the homology is so high that none of the other species responds to it.
Taking a look at the kinds of reagents available and the intended use of the final antibody will be important in deciding how the test bleeds and fusion will be screened to select the antibodies of interest and rule out the irrelevant and nonspecific hybridomas that grow out on the fusion plates. This should be a high-throughput assay that can handle the large number of samples that will be generated. It should also reflect the intended use of the antibody. You cannot assume that an ELISA-positive antibody will work by western blot or immunohistochemistry.
Finally, the immunization schedule needs to be decided on. A common immunization protocol might be as follows (Fig. 1):
-
1. For each mouse, mix 250 µL of antigen solution with 250 µL of Complete Freund's adjuvant. Inject six BALB/c female mice i.p.
-
2. After 14 d, repeat the injections, but use Incomplete Freund's adjuvant.
-
3. Collect tail bleeds from immunized mice on Day 24. Do 1:5 dilutions in PBS, and test all samples by comparison with similar dilutions of normal mouse serum in a dot blot.
-
4. On Day 35, inject all animals i.p. with Incomplete Freund's.
-
5. Day 45: Do tail bleeds and test by dot blot. All serum samples should be checked by immunoprecipitation against in vivo radiolabeled antigen preparation.
-
6. Day 56: Inject the best responder with 100 µL i.v. and 100 µL i.p. All others get intraperitoneal injection with Incomplete Freund's.
-
7. Day 59: Fuse splenocytes from the best responder.
-
8. Day 66: The first positives should be observable.
If time is critical, a more rapid immunization protocol can be selected such as RIMMS, which is also a good choice for target proteins where conformational epitopes are present. Weak immunogens could benefit from higher dosage per immunization or more frequent immunizations. If the target protein is part of a mixture, like a cell lysate, or present on the surface of transfected cells, using a subtractive immunization protocol could be helpful.
The test bleed will let you know if you have chosen well or need to re-examine all of the above. If the titer is negative or too low, you might decide to simply reboost a few times and check the titer again. If it has not improved, changing the dosage or form of the immunogen, adjuvant, or both might help. Sometimes you just have to start over with a different species or strain. Just because the titer is weak does not necessarily mean that a good hybridoma will not be obtained from the fusion—only that it is less likely. Persistence and a little luck will often lead to success.
Troubleshooting, or “What Do I Do Now?”
Here we examine some of the possible pitfalls and solutions working with antibodies can present:
Project 1
Monoclonal antibodies were needed for flow cytometry. The target protein was run through immunogenicity prediction software. Based on the results, two highly immunogenic regions were selected as immunogens. Peptides were synthesized (15-mer and 22-mer), bound to KLH (for immunization) and bovine serum albumin (for ELISA screening). A group of five BALB/c mice were immunized every 2 wk (s.c. and i.p.). After three injections, sera were collected (Day 10) and an ELISA titer was determined. The titer was greater than 1:100,000 above an irrelevant protein for most of the mice. The decision was made to fuse a mouse. It was rested 3 wk, then i.v.-boosted with peptides with spleen removal 3 d later for fusion. ELISA screening of the fusion produced more than twenty hybridomas producing monoclonal antibodies specific to one peptide or the other. Unfortunately, none of the resulting monoclonal antibody recognized the whole, native protein by ELISA or flow cytometry. What went wrong?
Nothing went wrong. This is always an issue when using peptides as immunogens. They have no secondary structure or posttranslational modifications that are present in the native form. If the targeted epitopes were fairly linear and unmodified, then the antibodies might work on western blot, but in this case, they are needed for flow cytometry.
The next step should be to re-examine the form of the immunogen. It needs to be in a form that will generate the desired antibody; proper folding is important. A recombinant protein that is folded correctly (i.e., has biological activity) would work. Immunizing with a transfected cell line would make screening more difficult but could also be successful. Genetic immunization was not an option for this project but could also have been considered.
Project 2
A monoclonal antibody is needed for immunohistochemistry of paraffin-embedded tissue sections. None of the commercially available antibodies work well. Because of cost considerations, a synthetic peptide (KLH-conjugated) was made to a very hydrophilic region on the peptide that was not believed to have had much in the way of posttranslational modifications. Standard immunizations of four BALB/c mice showed a reasonable titer by ELISA on the synthetic peptide versus an irrelevant peptide. The sera were also screened by immunohistochemistry. The slides were inconclusive. There seemed to be a slight positive response, but the background was very high. Flow cytometry using overexpressing transfected cells showed specific reactivity compared with mock-transfected (vector alone) cells. A decision was made to try a fusion to see if the background issues could be solved using a monoclonal antibody. The fusion went well, producing many flow cytometry–positive hybridomas, none of which produced the desired immunohistochemistry reactive antibody. Either there was no reaction or the background was unreasonable.
In this case, the flow cytometry data suggests that the peptide did generate antibodies that recognize the native protein, or at least the transfected version. So why did it not work on the paraffin sections? Closer examination of the form of the antigen that the required antibody needed to bind was important. Tissues were dehydrated and fixed with paraformaldehyde, then embedded in paraffin before being sectioned onto slides for immunohistochemistry. This elaborate process can induce changes in proteins. Aldehyde groups are added and cross-linking occurs. The dehydration process can change a protein's structure by removing water molecules in proximity to the protein. These changes can make the difference between an antibody recognizing a protein or not. To account for some of these changes, the transfected cells were fixed with paraformaldehyde followed by treatment with Tris buffer to cap off any reactive aldehyde groups. The fixed cells were thoroughly washed and used as immunogens. Mice were immunized, and the sera were screened by flow cytometry on native, fixed, and mock-transfected cells. The mice needed two rounds of immunizations (five injections total) until the fixed cells showed a convincing positive response. A fusion was performed, screening by flow cytometry as well. Although only five positive hybridomas were identified, one provided us with the desired reactivity by immunohistochemistry.
Project 3
The goal was to generate a monoclonal antibody that bound to a cell-surface receptor that also happened to be a GPCR (7-spanner). Furthermore, the antibody not only needed to bind to the receptor but needed to block ligand binding as well. The structure of the receptor was divided up into three extracellular loops plus an amino-terminal tail. Peptides were synthesized for each of these extracellular regions and used to immunize mice individually or as a cocktail containing all four peptides. Transfected CHO cells with a surface-expressed HIS tag were also made for use in flow cytometry–screening purposes. Antibody titers were weak to moderate by ELISA using the individual peptides and reaching a plateau where no further immunizations produced any increase. Flow cytometry with the transfected cells was sketchy to negative.
Giving the project more thought, it was decided to add linker sequences of alanine residues to space the loops out in the same spatial orientation they would be in the native GPCR. The old groups of mice and a new group were immunized. The titers did improve by ELISA but not significantly by flow cytometry. A fusion was tried. This generated antibodies to the three loop peptides, but all were negative by flow cytometry on the transfected CHO cells.
Because simply linking the peptides together did not sufficiently mimic the native conformation, the mice were immunized with transfected cells. To help with screening, a transfected HEK293 cell was made with a surface-expressed c-myc tag. A group of mice was immunized with either CHO or 293 cells. When bleeds were taken 7 wk later, all the mice were producing antibodies to the cells with a slightly higher response to the transfected cells than the mock-transfected cells. This looked initially promising, but, again, the titers remained low even with repeated immunizations. The GPCR expression was never thought to be very high, using the surface tag expression as an indicator. Perhaps there was not enough receptor to induce a good antibody response?
This is when an interesting piece of data came to light. The CHO cells were used in a fluorometric imaging plate reader (FLIPR) assay looking at calcium flux. Native B cells showed a spike when the ligand was introduced. The transfected CHO cells did not, nor did the transfected 293 cells. This indicated that the transfected cells did not express an active GPCR. An explanation for this could be that the transfected GPCR was not properly folded. Four or five different cell types were transfected before a pre-B cell was used that worked in the FLIPR assay.
New mice were immunized with these pre-B-transfected cells, but titers were still low. The flow cytometry data were weakly positive, but expression of surface GPCR was still weak as well. The bleed sera were tested in the FLIPR assay and were able to block binding of the ligand to the receptor out to 1:1000 dilution. The mouse that exhibited best blocking activity was selected for a fusion. Screening was initially by flow cytometry with confirmation by FLIPR assay (ligand-blocking activity). Three hybridomas were finally identified.
Project 4
An Escherichia coli–derived recombinant protein with a Gst tag was provided for generating monoclonal antibodies for use in immunoprecipitation. A group of five BALB/c mice was immunized using a standard protocol and bled for titer. The ELISA titer on the recombinant-Gst protein versus irrelevant-Gst protein indicated a high antibody response, but it appeared to be almost entirely directed toward the Gst-tag. Continued immunizations made the problem worse. Untagged protein or protein with a different tag was unavailable. A fusion was tried, but all the reactive hybridomas were directed against Gst.
Immunizing mice with the untagged protein or the protein with a different tag would be best. The test bleed serum could even be screened, using it to determine if there is really an antiprotein response that is being masked by the huge anti-Gst response. Because this was not an option here, a different strategy was needed.
It was decided to try using a subtractive immunization strategy in which new mice would be immunized with Gst-irrelevant protein, then treated with cyclophosphamide to kill off rapidly dividing B and T cells reactive toward Gst. After two rounds of treatment, the mice showed a 60%–70% drop in anti-Gst response. The mice were then immunized with the recombinant protein-Gst tagged (3×) and bled again. Reasonable titers were now observed toward the protein of interest.
In parallel, the old group of mice was also boosted with irrelevant protein-Gst and treated with cyclophosphamide. There were casualties: Of the remaining four mice in the group, two died during the subtraction attempt. The remaining two showed a decrease (∼20%) in serum titer toward Gst, but it was still significantly high, especially compared with the new group of mice.
Fusions were performed with a mouse from the old and, subsequently, the new mouse group. The fusion from the old mouse group produced two protein-specific hybridomas and fifteen Gst-specific hybridomas. The fusion using the new mouse had twelve protein-specific hybridomas but only three Gst-reactive hybridomas. It seems that the subtractive method is significantly more effective as a pretreatment.
Project 5
BALB/c mice were immunized with a peptide-KLH in Freund's adjuvant (s.c. and i.p.). The initial test bleed after three boosts had a very low titer (<1:300) by ELISA. The mice were reboosted with recombinant protein another three rounds (two boosts each) without any significant rise in titer, even alternating immunizations between peptide-KLH and peptide-OVA. The response had reached a plateau, and the level was too low for a successful fusion.
It was decided to try one more round of immunization, this time using a different adjuvant (Hunter's TiterMax Gold) and more subcutaneous sites than in the initial immunizations. The results were not dramatic, but the titers did finally increase to 1:1200. Another round of immunization using Ribi adjuvant brought the titer up to 1:6000, and a fusion was performed. There were two positive hybridomas identified that were specific.
Footnotes
-
From the Antibodies collection, edited by Edward A. Greenfield.